The presence of cerebral macrovasculopathy as detected by transcranial Doppler (TCD) exposes children with sickle cell anemia (SCA) to a high risk of stroke, preventable by chronic transfusion or stem cell transplantation (SCT). However, long-term outcomes of stenosis have not been well described. The Drepagreffe trial (NCT01340404) was a prospective trial comparing cerebral vasculopathy outcome after SCT vs standard-care in children with abnormal TCD with or without stroke history. Results from the whole population have recently been reported (Bernaudin et al, JAMA 2019). The decrease in velocities was significantly higher after SCT than standard-care (p<0.001) at 1 and 3-year, but the stenosis score was not different between both treatment groups. The aim of the present study was to determine stenosis outcome as a function of stroke or no-stroke history in both treatment groups using detailed post-hoc analysis.
Sixty-seven SCA-children on chronic transfusion for abnormal-TCD history were enrolled (Dec-2010/June-2013) in this prospective trial with two treatment groups defined by the random-availability of having a matched-sibling donor (MSD). Thirty-two with MSD were transplanted while 35 without MSD were maintained on chronic transfusion for at least one-year and eventually switched to hydroxyurea thereafter if no stenosis and normalized velocities. Cerebral and cervical magnetic-resonance angiography (MRA) was systematically performed at enrollment, and 1- and 3-year post-enrollment. Stenosis was defined as a narrowing ≥25%. The MRA stenosis-score, was calculated as the weighted sum of the scores in the 8 assessed cerebral arteries (right and left middle cerebral (MCA), anterior (ACA), internal carotid (ICA) and extracranial internal carotid arteries (eICA)), with 0 = stenosis, 1 = mild stenosis (25-49%), 2 = moderate stenosis (50-74%), 3 = severe stenosis (75-99%), and 4 = occlusion.
All 67 patients were alive at 3-year, and the 32 transplanted patients successfully engrafted. No stroke or recurrence occurred during the follow-up. No chronic-GVHD was observed.
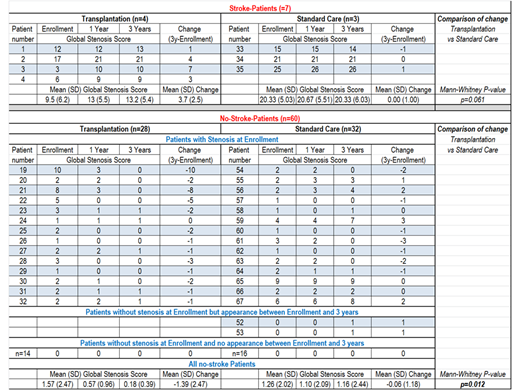
Among the 7 patients with stroke-history, all had stenosis at enrollment and the stenosis score increased in the 4 transplanted patients, but always in the arteries with previous stenosis and those feeding ischemic territories, while stenosis score remained mostly stable in the 3 patients maintained on chronic transfusion,. However, the difference between treatment groups was not significant (p=0.057).
Among the 60 stroke-free patients at enrollment, 28 with MSD were transplanted while 32 without MSD were maintained on chronic transfusion. At enrollment, 28 patients (14 patients in each treatment group) had stenosis. At 1-year, 9 patients in the SCT group had stenosis, whereas in the transfusion/standard-care group, 10 had stenosis. At 3-year, 5 patients in the SCT group had stenosis, while 10 still had stenosis in the standard-care group. Moreover, 2 patients, who had no stenosis at enrollment, developed one stenosis between 1 and 3-year, despite chronic transfusion in one case and after switch to hydroxyurea in the other. In another patient, stenosis had disappeared on chronic transfusion at 1-year, although it reappeared at 3-year after a switch to hydroxyurea. In the SCT group, no worsening of stenosis was observed, and stenosis improved in 13/14 and was stable in one; in contrast, worsening of stenosis score was observed in the standard-care group in 6 patients on chronic transfusion (p=0.035), The stenosis-score between enrollment and 3-year improved more significantly in the SCT group (mean (SD): -1.39 (2.47)) than in the standard care group (-0.06 (1.18)); (p=0.012).
Conclusions: This prospective trial reporting the outcome of stenosis in stroke and stroke-free SCA-patients with a history of abnormal-TCD shows a trend to worsening of the stenosis-score after SCT in stroke-patients, but no stroke recurrence; in contrast, in stroke-free patients, stenosis outcome was significantly better after SCT and with better prevention of stenosis occurrence than on standard care. These results support early recommendation of SCT in children with a history of abnormal-TCD and an MSD.
Verlhac:Addmedica, Paris: Other: Financial Support; Bluebird Bio: Consultancy. Brousse:bluebird bio: Consultancy; Add medica: Consultancy. De Montalembert:Addmedica: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees. Thuret:BlueBird bio: Other: investigators for clinical trials, participation on scientific/medical advisory board; Celgene: Other: investigators for clinical trials, participation on scientific/medical advisory board; Novartis: Other: investigators for clinical trials, participation on scientific/medical advisory board; Apopharma: Consultancy. Bernaudin:GBT: Membership on an entity's Board of Directors or advisory committees; AddMedica: Honoraria, Other: Help for travel to meeting; BlueBirdBio: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal