Background: Glucose transporter 1 (GLUT1) is a ubiquitously expressed protein highly expressed on the surface of erythrocytes. As a member of insulin signaling pathway, GLUT1 is responsible for basal and growth factor-stimulated glucose uptake. The insulin signaling pathway is involved in several different biologic functions including cellular metabolism, energy regulation, cell cycle control, and stress response. We have previously identified several components of the insulin signaling pathway, including FOXO3, AMPK, and IGFBP3, to be associated with fetal hemoglobin (HbF) levels in erythroid progenitor cells from sickle cell disease (SCD) patients. We have shown that metformin, a FOXO3 and AMPK activator, induces HbF in vitro. Furthermore, our preliminary data of metformin clinical trial in patients with SCD suggests that metformin can induce HbF in vivo. Studies in non-erythroid cells have reported that metformin increases production and surface expression of GLUT1; increase in the activated form of both AMPK and FOXO3 is also associated with elevated GLUT1 surface expression and its activation. Given these interesting associations, we hypothesized that GLUT1 levels may be associated with HbF levels in patients with SCD. To test this, we measured the expression levels of GLUT1 on the surface of red blood cells (RBC) from patients with SCD and investigated its correlation with hematologic indices.
Methods: Applying a receptor binding domain labeling technique using anti GLUT1 antibody (Metafora Biosystems), we quantified GLUT1 expression measuring its geometric mean fluorescence intensity index on the surface of the RBCs by flow cytometry (Attune NxT). GLUT1 and CD71+ expression was measured on peripheral blood samples collected from 13 pediatric patients with HbSS (all on hydroxyurea, none on transfusion therapy) under an IRB approved protocol from Baylor College of Medicine. Patients ranged from 4 to 21 years of age; 52% were male. 4 HbAA normal donors were also analyzed, ages 28 to 43 years old, 50% male. HbF levels were obtained on the same date of collection by HPLC (Agilent, 1260 infinity-2). Complete blood count with differential and absolute reticulocyte count (ARC) was measured by ADVIA-120 hematology analyzer (Siemens). Flow cytometry data was analyzed by FlowJo software. P-values were calculated using Student's t-test.
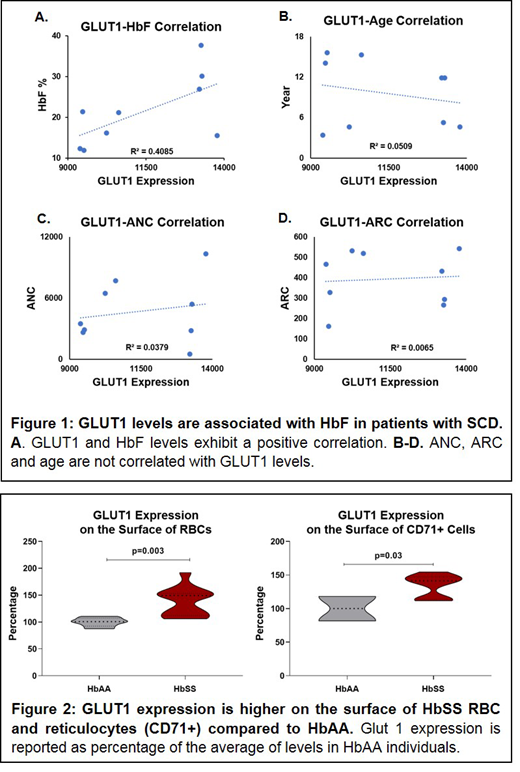
Results and conclusions: We identified a strong positive correlation between GLUT1 expression and HbF on the surface of hydroxyurea treated HbSS RBCs, R2=0.41. Possible variables contributing to this correlation are HU treatment differences, patient age, and RBC stage of maturation. However, 1) there was no correlation between GLUT1 levels and absolute neutrophil count (ANC), suggesting that variations in HU usage did not contribute to the association; 2) there was no association between patient age and GLUT1 levels; 3) there was also no correlation between GLUT1 levels and ARC or %CD71 positivity, suggesting that the GLUT1:HbF correlation was not due to more early stage erythroid cells with higher HbF levels due to a maturation arrest (Figure 1). GLUT1 expression was significantly higher on the surface of HbSS RBCs and CD71+ cells compared to HbAA (Figure 2); this is possibly due to the increased metabolic demand for glucose in the sickle RBC, but may also impact the basal HbF level of the sickle RBC. We hypothesize that cells with higher HbF levels have higher levels of activated AMPK, which activates FOXO3, a positive regulator of HbF (Zhang et al., Blood 2018), consistent with a metabolic stress state. Metabolic stress leads to an increase in GLUT1, to facilitate glucose transport. Future work will explore potential causative relationships between GLUT1 levels and HbF, and determine whether pharmacologic manipulation of GLUT1 may increase HbF in patients with SCD.
Petit:Metafora-biosystems: Equity Ownership, Other: CEO and co-founder.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal