
INTRODUCTION
Intravenous iron therapy in children with iron deficiency anemia (IDA) has previously been limited to those with severe or refractory anemia. However, increased availability of intravenous iron preparations with improved safety profiles has increased its utilization in both the adult and pediatric patient populations. Ferric carboxymaltose (FCM) was approved for adult patients by the Food and Drug Administration (FDA) in 2014 and has lower rates of severe allergic reactions. However, literature on adult patients has reported the development hypophosphatemia in up 50% of those receiving the drug. In early stages, hypophosphatemia can lead to abnormal bone mineral metabolism and hypercalciuria. Rickets and osteomalacia can result after prolonged hypophosphatemia. Severe, prolonged hypophosphatemia (<1 mg/mL) is associated with neurologic, cardiopulmonary, muscular, and hematologic complications. Although not FDA approved for pediatric use, FCM is increasingly being utilized for IDA in children. Hypophosphatemia in children treated with FCM has not been formally evaluated or described. We sought to assess available phosphorus levels in children treated with FCM at a tertiary care center.
METHODS
This was a single center retrospective cohort study of all children who received FCM over the initial 2.5 year period for which the drug was added to our institution's formulary (November 1, 2016 through April 30, 2019). Pharmacy records of all FCM infusions administered over this period were obtained. Medical record numbers of all patients who received an infusion were then searched for any available phosphorus testing. Patients were included in the review if they had phosphorus laboratory results available from 1 to 6 weeks post-receipt of FCM and were less than 21 years of age at the time of infusion.
Hypophosphatemia was defined as a phosphorus level below the lower limit of normal for age as delineated by our institution's central laboratory. Changes in phosphorus levels were calculated in those patients in whom pre-infusion phosphorus levels were also available within the 4 weeks prior to drug infusion. When multiple phosphorus levels were available within the post-infusion window, the lowest value was selected to calculate changes in levels. The electronic medical record (EMR) was reviewed in all patients in whom hypophosphatemia was identified to assess for administration of supplemental phosphorus, as well as other clinical factors that may affect phosphorus levels such as the administration of total parental nutrition (TPN) and/or renal medications (i.e. furosemide), as these are known risk factors for hypophosphatemia.
RESULTS
From November 1, 2016 through April 30, 2019, 1,081 infusions of FCM were administered in 656 patients. Post-infusion phosphorus testing was available in 165 patients in whom 247 infusions were administered (range 1 to 6). Patients' median age was 4.6 years (range 4 months to 20.6 years) and 56% (n=92) were female.
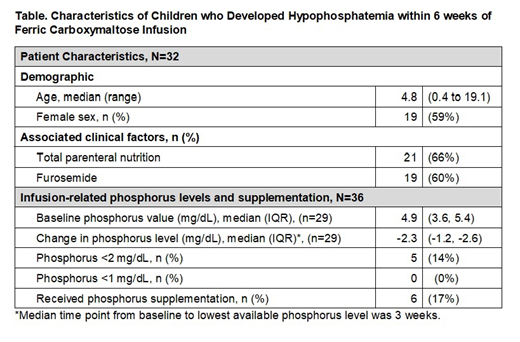
Hypophosphatemia occurred after 36 (15%) infusions in 32 unique patients (19%). Six patients (19%) received potassium phosphorus supplementation (Table). In the 23 patients in whom phosphorus testing was available at 6 weeks, 15 (65%) continued to have phosphorus levels below the normal value for age. Of the entire cohort, pre- and post-infusion phosphorus levels were available relative to 197 infusions (80%) in 136 unique patients (82%). The median change in phosphorus was -0.6 mg/dL (IQR -0.1, -1.6).
CONCLUSIONS
The assessment of serum chemistries, including phosphorus, is not routinely performed in otherwise healthy children with iron deficiency. While over 650 children received over 1000 infusions of FCM during a 2.5 year period, only 165 patients had phosphorus testing available during the stated time frame of the infusion. In those in whom testing was available, and in which hypophosphatemia occurred, the majority were patients admitted to the hospital with co-morbid conditions or complex clinical care for which phosphorus levels may be affected.
Our center has developed a clinical protocol to obtain baseline phosphorus levels in all children in whom intravenous FCM is being considered. Post FCM therapy, phosphorus monitoring is being performed to better identify those patients who may be at risk for hypophosphatemia and in whom phosphorus supplementation may be indicated.
Ferric carboxymaltose is FDA approved for treatment of iron deficiency in adults. This presentation will discuss the use of ferric carboxymaltose to treat iron deficiency in pediatric patients.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal