
Introduction
Environmental and genetic factors may lead to iron accumulation, causing irreversible organ damage. Homozygosity for the C282Y (C282Y +/+) and compound heterozygosity for the C282Y and H63D (C282Y-H63D) mutations of the HFE gene are associated with susceptibility to iron overload (IO). However, their clinical and biochemical expression is heterogeneous, with some patients showing only an increase of transferrin saturation (TSAT) for life, and others developing severe liver disease at a young age.
Rarely, IO occurs in subjects without HFE-mutations or other acquired factors (e.g. alcohol intake, hemolysis, etc.). In these cases, non-HFE hemochromatosis is suspected, but the diagnosis is challenging, based on invasive (i.e., liver biopsy) or poorly available (i.e., Next-Generation Sequencing) approaches.
A defective production of the iron regulatory hormone hepcidin is the key pathogenetic factor in hereditary hemochromatosis, irrespective of the gene involved, but extensive studies evaluating its potential diagnostic role are still lacking.
This project evaluated hepcidin levels in a large subpopulation from the Cooperative Health Research In South Tyrol (CHRIS) study. Here we explored in particular hepcidin levels in subjects with altered iron status parameters, and their role in the identification of subjects at major risk of developing IO.
Patients and Methods
Study Population. The CHRIS study is a population-based study carried out in South Tyrol (Northern Italy), whose general aims are reported in detail elsewhere (Pattaro C, J Transl Med 2015). Blood samples were tested for several biochemical and genetic parameters, including those related to iron status, such as TSAT, ferritin, and C282Y and H63D mutations.
Hepcidin was measured in stored aliquots by a recently updated and validated mass spectrometry-based method in tandem with liquid chromatography (LC-MS/MS), able to distinguish the active hepcidin-25 isoform (Diepeveen LE, Clin Chem Lab Med 2019).
Results
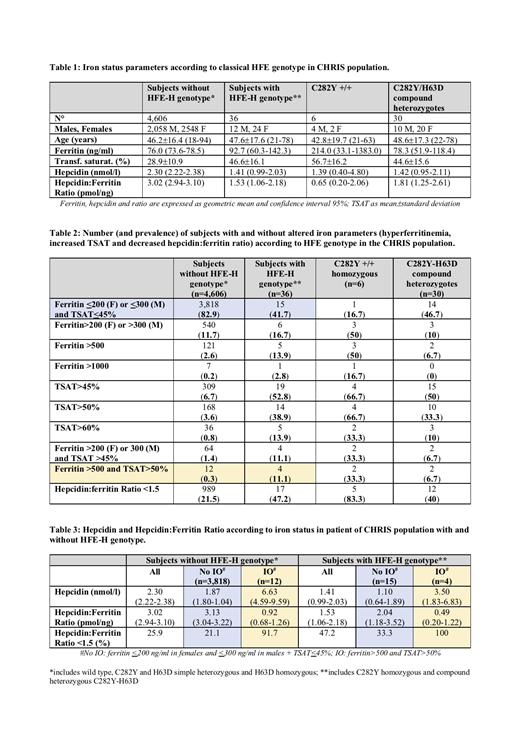
Among 4,642 participants, 6 were C282Y +/+ and 30 were C282Y-H63D (hereinafter defined as "HFE-H subjects"). HFE-H subjects showed ferritin levels only slightly higher than those with apparent wild-type HFE-H genotype (92.7 vs. 76.0 ng/ml, p=0.29), significantly higher TSAT (46.6 vs. 28.9%, p<0.0001) and lower hepcidin levels (1.41 vs. 2.30 nmol/l, p=0.016) (Table 1). Defective production of hepcidin was suggested by the reduced hepcidin:ferritin ratio (1.53 vs. 3.02 pmol/ng, p<0.0001), which was particularly low in C282Y +/+ (0.65 pmol/ng).
Table 2 shows the prevalence of subjects with altered iron parameters (hyperferritinemia and/or increased TSAT), according to the HFE genotype. As concern HFE-H subjects, hyperferritinemia (i.e. >200 or >300 ng/ml in females and males, respectively) was detected in 16.7%, increased TSAT (>45%) in 52.8% and both in 11.1%. A biochemical pattern suggestive of IO (ferritin>500 ng/ml and TSAT>50%) was seen only in 33.3% of C282Y +/+ and in 6.7% of C282Y-H63D, while 41.7% neither had hyperferritinemia nor increased TSAT, confirming the low penetrance of such genotype. Although HFE-H subjects displayed a tendency to increase hepcidin production according to iron deposits (mean level of 1.10 nmol/l in subjects without hyperferritinemia/increased TSAT vs. 3.5 nmol/l of subjects with IO), the hepcidin:ferritin ratio was significantly lower in phenotypically expressed HFE-H subjects (0.49 vs. 2.04 pmol/ng, p=0.014) (Table 3).
On the other hand, 540 participants (11.7 percent) without HFE-H genotype had hyperferritinemia, 64 (1.4%) had both hyperferritinemia and increased TSAT, and 12 (0.3 percent) had biochemical signs strongly suggestive of IO (ferritin>500 ng/ml and TSAT>50%). The latters had reduced hepcidin:ferritin ratio (0.92 pmol/ng), a value comparable to that of HFE-H iron loaded subjects (p=0.048). Whole Exome Sequencing data are available for the majority of CHRIS subjects included in this project and will be analyzed in detail in these subpopulations.
Conclusions
Our data suggest that the hepcidin:ferritin ratio may actually represent a useful indicator of hemochromatosis irrespective of the HFE genotype, possibly driving an optimal use of second level genetic test.
Girelli:Vifor Pharma: Other: honoraria for lectures; Silence Therapeutics: Membership on an entity's Board of Directors or advisory committees; La Jolla Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal