
Background: Iron deficiency anemia (IDA) is common in cancer patients due to blood loss and inflammation. Intravenous (IV) iron is commonly used in these patients as monotherapy or as an adjunct to erythropoiesis-stimulating agents as many do not tolerate, or adequately respond to oral iron. Over the last decade it has become apparent that hypophosphatemia may occur following some IV iron formulations. While initially considered a transient benign laboratory finding, a growing number of case reports have described treatment-emergent hypophosphatemia following IV iron administration as a safety consideration, especially in patients with normal renal function. Although hypophosphatemia may have clinical consequences, its diagnosis may be missed in the clinic due to initial nonspecific symptomatic presentation e.g., generalized weakness and fatigue. Patients with cancer, already at additional risk for hypophosphatemia for various reasons, including, bone metastasis, bone-modifying agents, and other drugs, might be at a higher risk for hypophosphatemia.
Objective: To evaluate the effects of IV iron treatment on the incidence of hypophosphatemia in the subgroup of patients with cancer who participated in a randomized, double-blind Phase 3 clinical trial (NCT02694978) that compared two IV iron preparations for the treatment of adults with IDA of any etiology (excluding dialysis-dependent CKD) and a history of unsatisfactory response to, or intolerance of oral iron.
Methods: Randomization was 1:1 to ferumoxytol (FER), administered as two 510 mg doses, or ferric carboxymaltose (FCM), administered as two 750 mg doses both delivered as an IV infusion over at least 15 minutes on days 1 and 8. Serum phosphate and other clinical laboratory values were measured at baseline, days 8 (prior to dose 2), 15, and 35. Data from patients with a recent or coincident diagnosis of cancer were extracted for post-hoc analyses. Within-group changes from baseline in phosphate levels were analyzed with paired t-test; between-group differences were analyzed using ANCOVA adjusted for baseline value. The overall incidence and visit-specific rates of severe hypophosphatemia (CTCAE Grade 3; <2mg/dL) were compared between treatments using Fisher's exact test. Risk factors for incident hypophosphatemia were explored with multivariable logistic regression that included randomized treatment group and baseline clinical factors as candidate predictors.
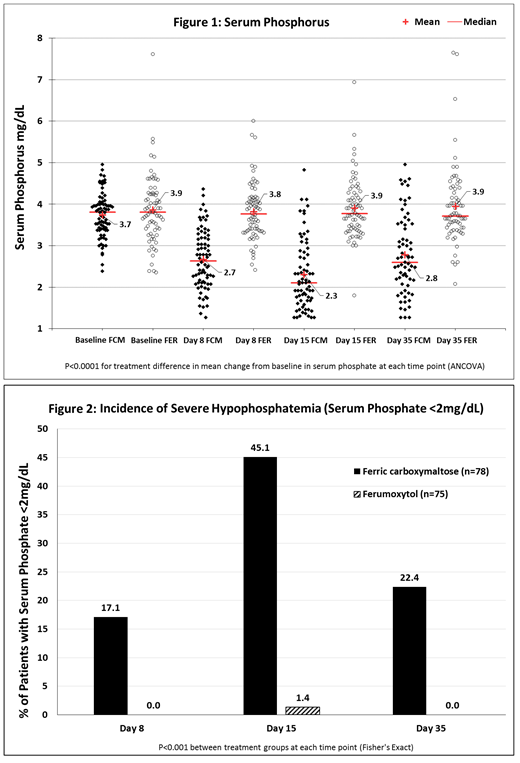
Results: The overall study safety population of 1,997 randomized patients who received any amount of study drug included 153 patients with cancer (78 FCM, 73 FER, 62.1% female; mean age 65.5 ± 13.2 years, mean baseline Hgb 10.5 ± 1.3g/dL, GFR 69.9 ± 32.4 mL/min/m2). Baseline serum phosphate was ≥2.4 mg/dL for all patients in the cancer subgroup, and mean values were similar between treatment groups (FCM 3.74 ± 0.53 and FER 3.86 ± 0.81 mg/dL).
Mean serum phosphate level in the FCM group decreased significantly at each time-point compared both to baseline and to the FER group (P<0.0001; Fig. 1), whose mean phosphate remained unchanged. The incidence of treatment-emergent severe hypophosphatemia (<2mg/dL) was higher in the FCM group compared with the FER group, both overall (46.1% vs. 1.4%, p<0.0001) and at each time point (P<0.001; Fig. 2), peaking in frequency at week 2, and remaining at 22.4% for FCM at week 5.
In a logistic regression model including factors for renal function, severity of anemia, weight, bisphosphonate use, and baseline phosphate level, the only independent predictors of hypophosphatemia were baseline phosphate level (OR 4.3, 95% CI 1.5 to 12.1, for each 1 mg/dL decrease, P=0.006) and treatment with FCM (OR 120.1, 95% CI 12.2 to 1182.1 vs. FER, P<0.0001).
Conclusion: Post hoc analyses of data from a Phase 3 clinical trial showed that mean serum phosphate decreased significantly in anemic cancer patients following FCM, but not FER, starting as soon as 8 days following the first 750mg dose, and did not return to baseline level by the end of the study period. This resulted in hypophosphatemia <2 mg/dL that remained unresolved in some FCM patients through the end of the 5-week study. While this study was not designed to assess the occurrence of symptomatic hypophosphatemia, the persistence of severe hypophosphatemia among FCM patients at the end of the 5-week study period suggests the need for monitoring serum phosphate following FCM usage in clinical practice.
Boccia:DSI: Speakers Bureau; Genentech: Speakers Bureau; AMAG: Consultancy; Amgen: Speakers Bureau; AstraZeneca: Speakers Bureau; Celgene: Speakers Bureau. Dahl:AMAG Pharmaceuticals, Inc.: Employment, Equity Ownership. Strauss:AMAG Pharmaceuticals, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal