Introduction
Thalassemia Syndromes (TS) are a group of inherited haemoglobin disorders characterized by different phenotype severity falling among heterozygote state, no transfusion dependent thalassemia (NTDT) and transfusion dependent Thalassemia (TDT) (Graffeo et al, 2018; Taher & Saliba, 2017).
Several factors, independently by genotype and globin chain unbalance, modulate the severity of ineffective erythropoiesis (Rivella et al, 2015).
Considering the complexity of this pathophysiology, one tool to evaluate patients on an individual basis is needed.
The aim of this study was to develop a severity scoring system with a view to initiate timely interventions that would prevent any further complications.
Methods
An International Health Repository (IHR) protocol was approved on May 25th, 2017 by the Italian Ethical Committee (EudraCT Code Numbers 2017-004457-17 and 143AOR2017). Subsequently, an International Working Group (IWG) on Thalassemia was formed and it met in Palermo, Italy, on September 15th and 16th, 2017. Indicators of phenotype severity thought to be most pertinent in defining disease severity were shared among the IWG based on their expertise as well as on expert opinion reported on literature. These data were collected and stored on IHR platform (www.sanitasicilia.eu/IWG ).
In order to define the prognostic score (PS) a retrospective multicenter case-control study was carried ahead.
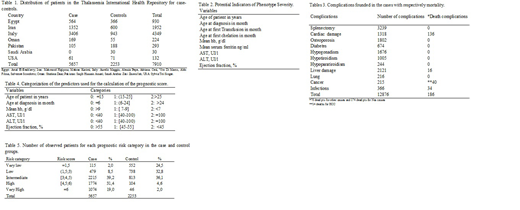
Overall, clinical findings of 7910 patients who attended the IWG centres from 1976 to 2018 were collected (Tab. 1).
The study was based on 5657 cases that developed complications and 2253 controls that didn't develop any complications. The term "patient" was used both for cases and for controls.
The variables used for the scoring system development were reported in Table 2.
The PS was built using a Conditional Logistic Regression Model (CLRM) (DW. Hosmer, Jr., 2013).
The full data-set was split into two data-set containing Group A and Group B patients.
Group A included transfused TDT and NTDT patients, while Group B included only no transfused NTDT patients. This was necessary because of the variable "age at first transfusion" was absent in no transfused patients. The two PS will refer as Score A and Score B, respectively.
The Stata 12 (StataCorp, College Station, TX, USA) was used for all analyses.
Results
Overall, the analysis was performed on 5657 cases (2812 Females, age at follow up 34.7±12.9) with a mortality of 8.5% and 2253 controls (1136 Females, age at follow up 22.1±12.8) with a mortality of 1.9%.
Table 3 shows complications and deaths in the full data set. Moreover, it even suggests, as single patient may have more than one complication and that the cardiac complications followed by the cancer and infection were the most common.
Table 4 shows the statistically significant variables for the calculation of the score.
The age of the patient and at first diagnosis, the mean Hb, AST, ALT and the Left Ventricular Ejection Fraction (LVEF) were the statistically significant variables at CLRM analysis, for the Group A.
The formula for the Score A=1.1x(Age)+0.9x(Age at first diagnosis)+1.3x(Hb mean)+1.006x(AST)+1.02x(ALT)+ 0.9x(EF).
The age of the patient, the mean Hb and the LVEF were the statistically significant variables at CLRM analysis for the Group B.
The formula for the Score B= 1.05x(Age)+1.001x(Hb mean) +0.9x(EF).
Finally, Table 5 shows the application of these formulas to the full data set, defining five well-distinguished prognostic categories (Very low, Low, Intermediate, High, Very high).
Conclusions
Following appropriate validation, we propose that the severity scoring system described here could be developed into a practical tool for widespread clinical application, not only to evaluate patient status and classify disease subgroups, but also to inform treatment decisions and monitor patient progress in response to therapy. Finally, the use of this scoring system may help to select appropriate candidate for innovative treatment.
Pepe:Chiesi Farmaceutici S.p.A., ApoPharma Inc., and Bayer: Other: No profit support. Vichinsky:bluebird bio: Consultancy, Research Funding; GBT: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Agios: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal