Introduction
The efficacy of rasburicase vs allopurinol for the prevention of hyperuricemia (HU) of tumor lysis syndrome (TLS) has been demonstrated in clinical trials, yet studies evaluating these therapies in real-world clinical practice are lacking. Here, we evaluate the real-world use and clinical impact of rasburicase and allopurinol for the prevention of chemotherapy-induced HU.
Methods
Data were collected from a proprietary aggregated Electronic Medical Records database containing data from 21 healthcare organizations and 26 million patients in the US. Inclusion criteria included patients at high risk (HR) of TLS (Table 1) and diagnosed with acute lymphocytic leukemia, acute myeloid leukemia, Burkitt's Lymphoma, chronic lymphocytic leukemia, or diffuse large B-cell lymphoma, and receiving chemotherapy between 01/01/2017 and 06/30/2018. Baseline measures were those within 6 months but closest to and before the start of chemotherapy. Patients were followed until death or 6 months from the start of chemotherapy. Statistical analyses included Student's t-test for continuous variables and Chi-squared, Fisher's exact, or McNemar's test for categorical variables. Multivariate analyses were conducted to assess association of variables and to generate scores for propensity score matching (PSM).
Results
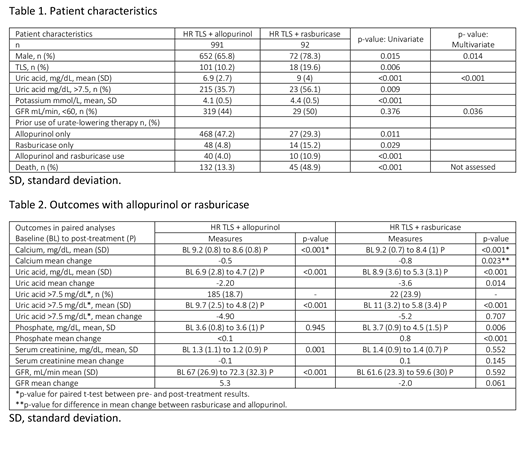
Of the patients who qualified for the study, 74% (991) received allopurinol, 7% (92 patients) rasburicase, and 19% (248) did not receive a urate-lowering therapy. Patients receiving rasburicase were more likely to be male, with pre-existing TLS at chemotherapy drug initiation, and previously treated with rasburicase or both urate-lowering therapies. The TLS risk population receiving rasburicase presented with higher mean baseline uric acid level (9.0 mg/dL) compared with patients receiving allopurinol (6.9 mg/dL), and over half had baseline uric acid levels >7.5 mg/dL (56% vs 35% for allopurinol). Multivariate analysis further indicated that a greater fraction of patients receiving rasburicase had renal impairment (glomerular filtration rate [GFR] <60 mL/min at baseline) compared with the population receiving allopurinol (Table 1). Treatment with rasburicase significantly decreased levels of uric acid and calcium and improved phosphate levels from baseline to post-treatment. These changes were significantly greater than observed in patients receiving allopurinol. In contrast, patients receiving allopurinol recorded improved GFR whereas changes in the rasburicase-treated population did not reach significance (Table 2). Follow-up varied for these treatment groups: 49% of patients at HR of TLS receiving rasburicase died within 6 months of the start of chemotherapy compared with 13% of allopurinol patients (p<0.001). PSM yielded small matched samples no longer representative of the severity of the condition of the rasburicase patients. No significant differences in rasburicase vs allopurinol efficacy were detected in these smaller samples.
Conclusions
Patients at HR of TLS and receiving rasburicase had higher uric acid levels at baseline, more renal impairment, and were more likely to have experienced TLS previously compared with those receiving allopurinol. Both rasburicase and allopurinol significantly decreased levels of uric acid post-treatment though the mean decrease was significantly higher in rasburicase patients. These data support previous clinical findings that rasburicase is highly effective in reducing uric acid, though observed use in the real-world setting is restricted to patients with increased sickness. These findings support the need for continued research of the outcomes associated with early recognition and prophylaxis with rasburicase for patients with moderate risk and HR features for HU of TLS.
Radtchenko:Sanofi: Research Funding. Lyons:Sanofi: Employment. Barnes:Sanofi: Employment. Milligan:Trio Health: Employment; AbbVie: Research Funding; Amgen: Research Funding; Gilead: Research Funding; Merck: Research Funding; Sanofi: Research Funding; Viiv: Research Funding. Drea:Sanofi-Genzyme: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal