Background: As part of system wide implementation of Patient Blood Management (PBM), transfusion guidelines were implemented at St. Luke's Medical Center, Advocate Aurora Health in Milwaukee, WI.
Methods: Beginning in 2015 Aurora Health Care made a platelet (PLT) count of ≤10K the standard threshold below which PLT transfusion is considered appropriate. Additionally, transfusing PLTs as single donor units with a PLT count between each unit was made standard. Education was provided to clinicians on the new standard including best practice advice in the transfusion ordering workflow. Data was gathered on institutional PLT use and individual clinician PLT ordering practices. Further education was provided based on the extent to which data showed conformity to the new standard.
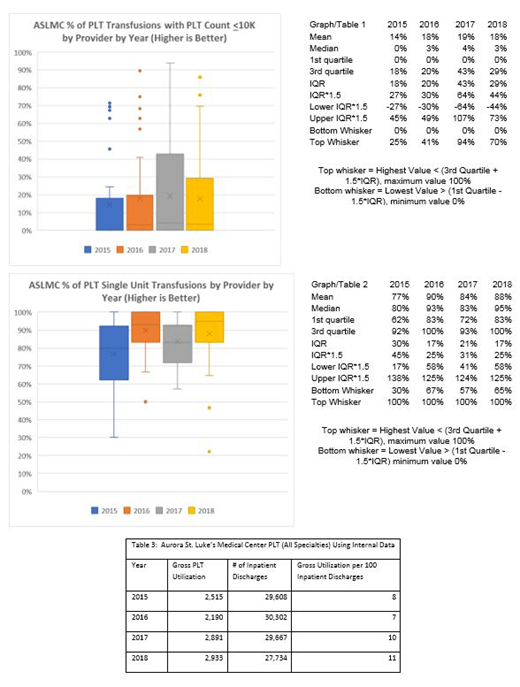
Results: During PBM implementation 2015-2018 despite an initial decline there was a modest overall increase in institutional PLT usage from 2515 to 2933 units. The percentage of transfusions ordered with PLT ≤10K rose from 21.6% to 31.9%. The percentage of transfusions ordered as single donor units increased from 82.5% to 91.5%. See Graph/Table 1 & 2 for further analysis of individual practitioner PLT ordering. See Table 3 for reporting period volume data.
Conclusions: Implementation of PLT transfusion guidelines was associated with an initial annual reduction in PLT usage, but annual PLT utilization did go up overall during the reporting period. Individual clinician transfusion practices overall conformed more with guidelines at the end of the reporting period than at the beginning. Changes in treatment guidelines for acute promyelocytic leukemia, i.e. target PLT >50K, during the reporting period may account for a rebound in PLT usage and a decrease in the percentage of transfusions ordered with PLT ≤10K in the later vs. the earlier years of the reporting period. By contrast transfusions continued a positive trend toward being given as single donor units even in the later years of the reporting period.
Thompson:Doximity: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Lynx Bio: Research Funding; BMS: Research Funding; VIA Oncology: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Adaptive: Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties: Myeloma reviewer; AbbVie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal