Background:
Patients living with cancer who develop venous thromboembolism (VTE) have a high risk of VTE recurrence, and traditional anticoagulants (low molecular weight heparin [LMWH] or vitamin K antagonists [VKAs]) are associated with significant treatment burdens. Rivaroxaban is a direct oral anticoagulant (DOAC) that may provide a more convenient treatment option for these patients.
Methods:
The COSIMO study was a multinational, prospective, non-interventional, single-arm cohort study designed to collect real-world data on patient treatment satisfaction and outcomes associated with rivaroxaban treatment following ≥4 weeks of LMWH/VKA therapy for the treatment of acute VTE in patients with active cancer. Here, we report on the secondary objectives, which were to provide descriptive analyses of clinical characteristics and patterns of use of anticoagulant treatment, and to assess the safety and effectiveness of rivaroxaban in this patient population.
Results:
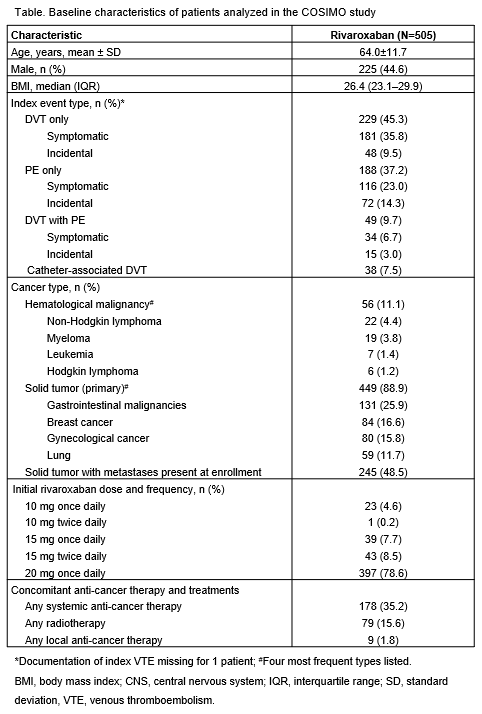
Overall, 505 patients were enrolled, and the qualifying venous thromboembolic event was deep vein thrombosis (DVT) only in 45.3% of patients, pulmonary embolism (PE) only in 37.2% of patients, DVT with PE in 9.7% of patients, and catheter-associated DVT in 7.5% of patients (Table). The majority of patients had solid tumors (n=449, 88.9%); 56 patients had hematological malignancies. The most common reasons to switch to rivaroxaban were patient preference/quality of life (n=310, 61.4%) and physician decision (n=174, 34.5%). A total of 150 (29.7%) patients were treated with concomitant chemotherapy and 79 (15.6%) received concomitant radiotherapy. Overall, 117 (23.2%) patients discontinued the study: 59 (11.7%) died, 21 (4.2%) withdrew consent, and 17 (3.4%) were lost to follow-up. 80.2% of patients were treated with rivaroxaban for at least 3 months, and most patients (78.6%) received rivaroxaban 20 mg once daily on study entry. Treatment-emergent adverse events (AEs) were reported: 312 (61.8%) patients had an AE (148 [29.3%] serious AEs), and 95 (18.8%) patients had a bleeding event reported, of which 18 (3.6%) patients had an adjudicated major bleeding event. Adjudicated symptomatic and incidental VTE recurrence occurred in 15 (3.0%) and 3 (0.6%) patients, respectively. Adjudicated other site thromboembolic events such as splanchnic or cerebral vein thromboses were symptomatic in 1 (0.2%) patient and incidental in 1 (0.2%) patient.
Conclusions:
Observed incidence rates of VTE and bleeding events in COSIMO were similar to previous studies of DOACs for VTE treatment in patients with active cancer (Young AM et al. J Clin Oncol 2018;36:2017; Raskob GE et al. N Engl J Med 2018;378:615).
Study governance
Bayer AG
Funding
The COSIMO study is funded by Bayer AG and Janssen Pharmaceuticals.
Trial protocol number
NCT02742623. Registered 19 April 2016.
Documented approval from appropriate independent ethics committees/institutional review boards will be obtained for all participating centers prior to study start. Patients were asked to provide signed informed consent forms before joining the study. Few patients have yet completed the study, and so no data are available to share.
Acknowledgements
Editorial assistance was provided by Kate Weatherall of Chameleon Communications Int. Ltd. with funding from Bayer AG and Janssen Scientific Affairs, LLC.
Maraveyas:Bristol-Myers Squibb: Honoraria; Bayer AG: Honoraria, Research Funding; Pfizer: Honoraria. Beyer-Westendorf:Pfizer: Honoraria, Research Funding; Bayer HealthCare: Honoraria, Research Funding; Boehringer Ingelheim: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding. Lee:Pfizer: Consultancy, Honoraria; LEO Pharma: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding. Mantovani:Daiichi Sankyo: Research Funding; Boehringer Ingelheim: Honoraria, Research Funding; Bayer AG: Honoraria; Fondazione Charta: Consultancy; Pfizer: Honoraria. De Sanctis:Bayer US LLC: Employment, Equity Ownership. Abdelgawwad:Bayer AG: Employment. Fatoba:Bayer AG: Employment. Bach:Bayer AG: Employment. Cohen:Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GlaxoSmithKline: Consultancy, Speakers Bureau; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; ACI Clinical: Consultancy; CSL Behring: Consultancy; Aspen: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVie: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Lifeblood: Other: advisor to Lifeblood: the thrombosis charity and is the founder of the European educational charity the Coalition to Prevent Venous Thromboembolism; Portola: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ONO: Consultancy, Membership on an entity's Board of Directors or advisory committees; Boehringer-Ingelheim: Consultancy, Speakers Bureau; TRN: Consultancy; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Temasek Capital: Consultancy; Boston Scientific: Consultancy; Guidepoint Global: Consultancy; Medscape: Consultancy, Speakers Bureau; McKinsey: Consultancy; Navigant: Consultancy; UK Government Health Select Committee: Other: advised the UK Government Health Select Committee, the all-party working group on thrombosis, the Department of Health, and the NHS, on the prevention of VTE; Leo Pharma: Consultancy; GLG: Consultancy; Johnson and Johnson: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal