Introduction: For patients with newly diagnosed multiple myeloma (NDMM) who are eligible for autologous stem-cell transplantation (ASCT), two standard of care (SoC) induction regimens are bortezomib/cyclophosphamide/dexamethasone (VCd) and bortezomib/thalidomide/dexamethasone (VTd), each followed by ASCT. While VCd and VTd are both treatment options according to international guidelines, treatment selection varies by country. Additionally, while some clinical studies have evaluated the efficacy and safety of these therapies, direct comparisons have been limited to response endpoints post-induction and post-transplant (Moreau P, et al. Blood. 2016;127[21]2569-2574; Cavo M, et al. Blood. 2014;124[21]197; Cavo M, et al. Leukemia. 2015;29[12]2429-31). Herein we describe real-world treatment patterns in the United States for patients with NDMM who are transplant-eligible, and report results from a matched adjusted comparison to evaluate real-world long-term efficacy (overall survival, OS) for VCd +ASCT versus VTd +ASCT.
Methods: Data for the VCd and VTd real-world evidence (RWE) cohorts were identified from 3 US data sources collectively covering the period January 2000 to March 2017: the OPTUM™ Commercial Claims database, the OPTUM™ Integrated (CLAIMS+EMR) database, and the Surveillance, Epidemiology, and End Results (SEER)-Medicare Linked database. RWE data were from patients with an index MM diagnosis on or after 1 January 2007, medical prescription coverage in place at diagnosis, no prior malignancies in the 1-year period prior to index diagnosis, a 1-year look-back period prior to index diagnosis, received ≥1 line of therapy, and received stem cell transplantation with induction as frontline treatment. The Kaplan-Meier method and Cox proportional hazard model compared outcomes with and without adjustments for baseline characteristics (age, sex, renal impairment, and anemia) and induction treatment duration; comparisons were also conducted with inverse probability of treatment weighting (IPTW).
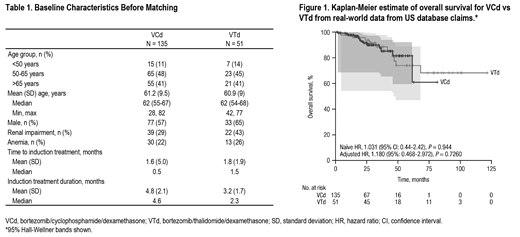
Results: Analysis of RWE from the United States demonstrated that bortezomib (V)-based regimens were the most common induction treatment (together accounting for approximately 75% of therapies), with bortezomib/lenalidomide/dexamethasone (VRd) being the most common (31%). Use of VCd (13%) and VTd (5%) was limited. Comparisons were conducted for VCd (n = 135) and VTd (n = 51). Baseline characteristics were generally similar between groups, except for fewer male patients in the VCd group than the VTd group (57% vs 65%), and lower rates of renal impairment in the VCd group than the VTd group (29% vs 43%; Table 1). The naïve and adjusted comparisons of OS for VCd versus VTd therapy showed these treatments were not statistically different (adjusted hazard ratio, 1.180 [95%: 0.468-2.972]; P = 0.7260; Figure 1). The IPTW method generated similar results.
Conclusions: Real-world data from the United States show that V-based induction regimens are the most commonly used for treatment of patients with NDMM who are transplant-eligible. Results from the naïve, adjusted, and IPTW comparisons all showed that OS was not significantly different for VCd + ASCT versus VTd + ASCT. Survival data for VTd from RWE are generally consistent with VTd data reported in the recent phase 3 CASSIOPEIA study, although OS data from CASSIOPEIA remain immature (Moreau P, et al. Lancet. 2019;394[10192]:29-38).
Cote:Janssen: Employment, Equity Ownership. Kampfenkel:Janssen: Employment, Equity Ownership. Nair:Janssen: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal