Background: Autologous stem cell transplantation (ASCT) as consolidation post induction is standard of care for eligible multiple myeloma patients. Multiple strategies are employed for mobilization of stem cells in ASCT. Cyclophosphamide/GCSF is an effective standard regimen. However there are reported toxicities associated with cyclophosphamide including risk of febrile neutropenia. Since plerixafor was introduced in Canada, this mobilization agent has been increasingly used as needed with GCSF at Kingston Health Science Centre (KHSC), with elimination of cyclophosphamide. There is however high cost associated with plerixafor. To date, there is no standard mobilization regimen across Canada. This retrospective study evaluates mobilization and ASCT outcomes of multiple myeloma patients who had undergone stem cell mobilization with GCSF+/-plerixafor (GP) vs cyclophosphamide/GCSF+/-plerixafor (CyGP) at KHSC. The goal is to analyze the efficacy of each regimen balanced with adverse events to guide optimal mobilization regimen choice.
Method: Patient with multiple myeloma who had ASCT at KHSC were selected consecutively based on date of ASCT in a reverse chronological order starting from Jan 2019. From Oct 2015 to March 2018, CyGP was the mobilization regimen used at KHSC. From March 2017 to January 2019, cyclophosphamide was eliminated and GP was used. Both groups could have received plerixafor as needed based on Cancer Care Ontario funding criteria such as failure to achieve target CD34+ cell count. Retrospective chart review was conducted to collect data on demographics, plerixafor use, days of apheresis, total cell collected, rate of febrile neutropenia, length of transplant hospital stay, time to engraftment, and transfusion requirement. Chi-Square, t-test, or Mann Whitney methods were used for test of statistical significance where appropriate.
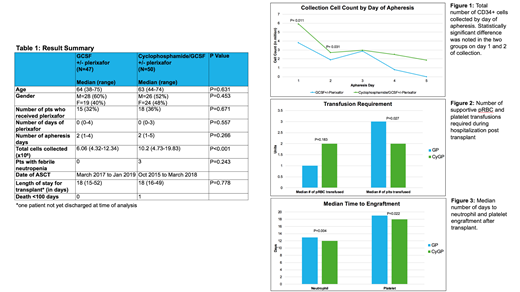
Results: Total 97 patients were included with 47 in the GP group and 50 in the CyGP group (Table 1). Average age was 64 and 63 respectively. 32% of patients in GP group and 36% of CyGP group required plerixafor rescue. No difference in the number of apheresis days were noted in the two groups. Total cell collection was significantly higher in the CyGP group (P<0.001). Day 1 and 2 cell collections were also significantly higher in the CyGP group (P<0.05) (Fig 1). The cell dose target varied in the CyGP group due to changing practices over time which is a confounding factor. There were no cases of febrile neutropenia in the GP group and 3 cases in the CyGP group (P=0.243). All 3 febrile neutropenia patients required hospitalization with 1 requiring ICU. There was no clinically significant difference in transfusion requirement during ASCT (Fig 2). Median time to engraftment of neutrophil and platelet after ASCT were less by 1 day in the CyGP group (P<0.05) (Fig 3), although there was no difference in length of transplant hospital stay.
Conclusions: Stem cell mobilization with CyGP resulted in significantly higher total collection cell count, and more efficient collection with higher yield on the first 2 days of apheresis. However, there was no difference in the total number of apheresis days. It also did not lead to less plerixafor usage and was associated with possibly higher rate of febrile neutropenia. There was no clinically significant difference in transplant outcomes in terms of transfusion support required, days to engraftment, or length of hospitalization for ASCT. Mobilization with GCSF+/-plerixafor without cyclophosphamide is a non-inferior regimen.
Bhella:Celgene: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal