Background: Around a third of patients with diffuse large B-cell lymphoma (DLBCL) either relapse following first-line (1L) chemoimmunotherapy (CIT) or are refractory to 1L CIT. Among patients with relapsed or refractory (R/R) disease eligible for second-line (2L) therapy, fewer than half survive beyond 5 years. Refractory DLBCL entails a poorer prognosis than relapsed DLBCL; however, literature on healthcare resource use (HRU) for these two populations is sparse. This study sought to describe HRU and healthcare costs in U.S. patients with relapsed and refractory DLBCL.
Methods: A retrospective claims analysis was conducted using the Optum® ClinformaticsTM DataMartTM database (01/2013-03/2018). Adult patients with ≥1 hospitalizations or ≥2 outpatient (OP) visits with an ICD-10-CM diagnosis code for DLBCL after 10/01/2015 were included. Patients were defined as (1) incident if they had no prior ICD-9-CM diagnosis code for unspecified DLBCL or primary mediastinal large B-cell lymphoma (PMBCL), or as (2) prevalent if they had a prior ICD-9-CM code for unspecified DLBCL or PMBCL. For incident cases, the index date was defined as the date of the first ICD-10 code for DLBCL or other lymphoma (after 10/01/2015); for prevalent cases, the index date was the date of the first unspecified ICD-9 code for DLBCL or PMBCL. Patients with an ICD-10 code for PMBCL at any time, or an ICD-9/ICD-10 code for Hodgkin lymphoma, multiple myeloma, and other selected lymphomas during the 12 months before the index date were excluded.
Patients with DLBCL were classified as relapsed if they initiated 2L ≥90 days after the last dose of the 1L therapy and as refractory if they initiated 2L <90 days after the last dose of 1L therapy. The 12-month period prior to the index date was defined as the baseline period. HRU (including hospitalizations, OP, ER, and other visits) and associated costs, including pharmacy costs, were evaluated per patient per year (PPPY) from the initiation of 1L up until end of data availability or end of continuous health plan enrollment.
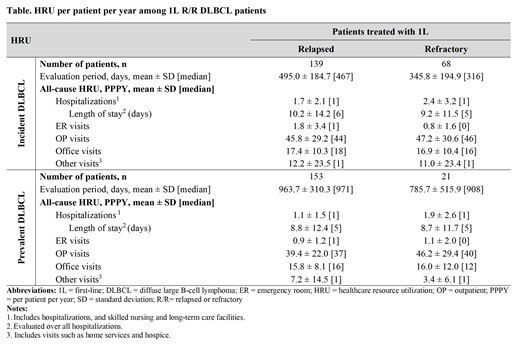
Results: A total of 139 and 68 incident DLBCL patients were identified as relapsed and refractory, respectively, with median (interquartile range [IQR]) age of 74 (68-81) and 72 (67-78), and mean Quan-Charlson comorbidity index score of 3.1 in both cohorts. The mean (SD) number of baseline all-cause hospitalizations was 0.35 (0.67) for relapsed and 0.40 (0.98) for refractory patients. Mean total baseline healthcare costs were $21,195 for relapse and $29,754 for refractory patients. R/R patients with prevalent DLBCL (153 relapsed; 21 refractory) had similar baseline characteristics.
In incident DLBCL patients, those with relapsed and refractory DLBCL were observed for 495 and 346 days, respectively. On average, 1.7 and 2.4 hospitalizations PPPY occurred in relapsed and refractory patients, respectively. The table shows the breakdown of HRU by type of visits. Although hospitalizations were qualitatively higher in refractory patient, mean ± SD total healthcare costs were in similar range (relapsed= $164,631 ± 115,503; refractory= $159,729 ± 102,442). Corresponding OP costs (relapsed= $99,748 ± 85,350; refractory= $86,505 ± 59,081) were mainly driven by DLBCL anti-cancer therapy costs and were qualitatively higher among relapse compared to refractory patients ($41,768 ± 46,135 and $29,454 ± 32,242, respectively).
In prevalent DLBCL, evaluation periods of relapsed and refractory patients lasted 964 and 786 days, respectively. Patients with relapsed DLBCL had 1.1 hospitalizations PPPY, and those with refractory DLBCL had 1.9 hospitalizations PPPY. Corresponding mean ± SD total healthcare costs were $112,653 ± 79,617 and $95,465 ± 50,167 PPPY, respectively. More pronounced results (i.e., higher rates of hospitalizations and corresponding healthcare costs for refractory compared to relapse patients) were observed in a sensitivity analysis when HRU and costs were assessed up to 6 months post-index date, which suggests that refractory patients are more likely to be hospitalized early on.
Conclusions: In this U.S. real-world study, patients with R/R DLBCL incurred substantial HRU and costs, thereby imposing a considerable burden on the healthcare system. There was a trend towards higher hospitalizations for refractory patients, suggesting a worse prognosis; however total healthcare costs were similar, offset by higher anti-cancer therapy costs among relapsed patients.
Yang:Merck & Co.: Employment. Laliberté:Janssen Scientific Affairs, LLC: Research Funding; Merck & Co., Inc.: Research Funding. Germain:Janssen Scientific Affairs, LLC: Research Funding; Merck & Co., Inc.: Research Funding. Raut:Merck & Co., Inc.: Employment. Duh:Analysis Group, Inc., a consulting firm that has received research funding from Shire, a Takeda company, to conduct this study: Employment; Shire: Research Funding; Merck: Research Funding. Sen:Merck & Co., Inc.: Employment. Lejeune:Merck & Co., Inc.: Research Funding. Desai:Merck & Co., Inc.: Employment. Armand:Merck & Co.: Employment, Membership on an entity's Board of Directors or advisory committees, Other: Travel fees, gifts, and others, Research Funding; Pfizer Inc: Research Funding; Otsuka: Research Funding; Bristol Myers Squibb Pharmaceuticals: Employment, Membership on an entity's Board of Directors or advisory committees, Other: Travel fees, gifts, and others, Research Funding; Roche: Research Funding; Dana-Farber Cancer Institute: Employment; Sigma-Tau: Research Funding; Affimed: Research Funding; Serventa: Research Funding; Infinity Pharmaceuticals: Employment, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal