Introduction: The treatment landscape for hemophilia A has evolved since the introduction of extended half-life (EHL) factor/non-factor VIII products, which allows for treatment personalization as per individual need, such as a less frequent infusion schedule and increased protection. Many different treatment attributes (e.g. safety, efficacy, and treatment burden) can influence patients' and caregivers' decisions in their choice of treatment. We used a discrete choice experiment (DCE) to elicit preferences from hemophilia A patients and caregivers for the treatment attributes of new EHL/non-factor products.
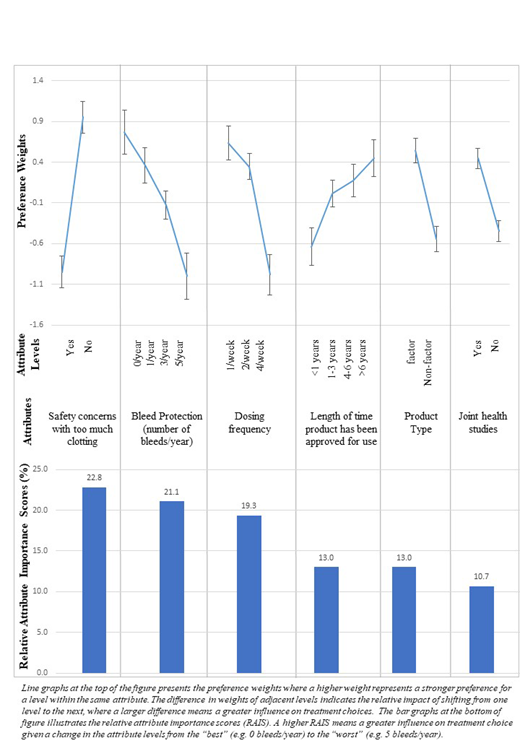
Methods: Adult patients and caregivers providing care for a young patient (<18 years) were recruited via a hemophilia A patient panel to complete an online survey with 2 components: (1) sociodemographic and treatment experience; (2) DCE with 12 treatment choice questions. Each question required respondents to choose between 2 hypothetical treatment profiles varying in terms of 6 attributes (Figure): safety concerns with too much clotting, bleed protection, dosing frequency, length of time the product has been approved for use, product type, and joint health studies. These attributes were selected based on a targeted literature review, 10 in-depth interviews with patients and caregivers, and clinical expert input. The results from the qualitative work have been previously published. The DCE survey was pilot tested with patients (N=3) and caregivers (N=3) for comprehensibility and revised prior to data collection. We used random parameters logit models to estimate preference weights and relative attribute importance scores.
Results: 113 patients (mean age: 35.5 years) and 96 caregivers (mean age of the child they cared for: 10.3 years) were included in the analysis. Among them, 88 patients (77.9%) self-reported having severe hemophilia while 81 (84.4%) caregivers reported that the child (<18 years) they cared for had severe hemophilia. Of these, 43 patients (38.1%) and 37 caregivers (38.5%) reported that their child with hemophilia used EHL/non-factor products. Among both patients and caregivers, the attributes from most to least important were as follows: (1) no safety concerns with too much clotting; (2) reducing annual bleeds from 5 to 0; (3) reducing weekly dosing from 4 to 1; (4) longer length of time the product has been approved for use (>6 years vs. <1 year); (5) factor product (vs. non-factor product), and (6) having studies demonstrating joint health improvement (vs. no studies) (Figure). The importance assigned to dosing frequency and bleed protection depended on the extent of improvement. For example, patients and caregivers valued having studies demonstrating joint health improvement more than they valued (a) a small improvement in bleed protection (i.e., 1/year to 0/year), or (b) a small improvement in dosing frequency (i.e., 2/week to 1/week). Lastly, further stratified analysis by patient and caregiver subgroups showed that patients viewed dosing frequency as the most important attribute influencing their treatment choices while caregivers valued safety the most. Among those who were not currently using EHL/non-factor products, the majority were willing to switch to EHL/non-factor products (81.4% of both cohorts). Bleed protection and dosing frequency were the most common reasons cited by respondents willing to switch treatments.
Conclusions: Besides safety, other attributes of products such as bleed protection and reducing dosing frequency had an important influence on hemophilia A patients' and caregivers' treatment choices in the DCE survey. The importance that patients and caregivers placed on these attributes also depended on the extent of bleed protection improvement or dosing reduction. Adult patients and caregivers making treatment decisions for their young patients may value dosing and safety of treatments differently. With more treatment options becoming available for people with hemophilia, it is important that patients and caregivers are well-informed about the comprehensive benefit-risk profile of different products to make the most optimal decisions to improve their current treatment and outcomes.
Joshi:Pharmerit International: Employment. Ng:Pharmerit International: Employment. Botteman:Alnylam Pharmaceuticals: Consultancy; Pharmerit International: Employment, Equity Ownership; Daiichi Sankyo: Consultancy, Research Funding. Li:Bioverativ, a Sanofi Company: Employment. Shah:Pharmerit International: Employment. Jain:Sanofi Genzyme: Employment, Equity Ownership. Lyn:Sanofi Genzyme: Employment. Su:Sanofi Genzyme: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal