Introduction: Although venous thromboembolism (VTE) is a well-known complication of cancer, predicting and mitigating the risks associated with VTE related to cancer remain a challenge.1 In 2008, Khorana et al. proposed an objective risk model that could be used to stratify patients into low, intermediate and high risk groups for VTE.2 There is also some evidence that a high KS is associated with increased mortality.3,4,5 This coding system, termed the Khorana score (KS), is based on widely available objective data and has been studied in academic teaching hospitals to assess risk of VTE. Here we examined the generalizability of the KS to risk of VTE in a community hospital setting and examined association with mortality.
Methods: A retrospective cohort study was conducted at the outpatient infusion clinic at Mount Auburn Hospital (MAH), Cambridge, MA. Patients aged 18 years or older undergoing chemotherapy for a new diagnosis of cancer, or undergoing new chemotherapy for disease progression or re-occurrence with an interval of >3 months from prior chemotherapy were included. Exclusion criteria included insufficient documentation, on clinical trials, surgery <2 weeks, past medical history of VTE and continuous anticoagulation. Patients were evaluated for a 6 month period beginning from January 2016 to December 2017. Data was collected and analyzed in STATA v16 using simple logistic regression. Primary study endpoints were VTE or mortality at 6 months. We also examined mortality at any time during or after the study period till May 2019 (2.5 years). This study was approved by IRB at MAH.
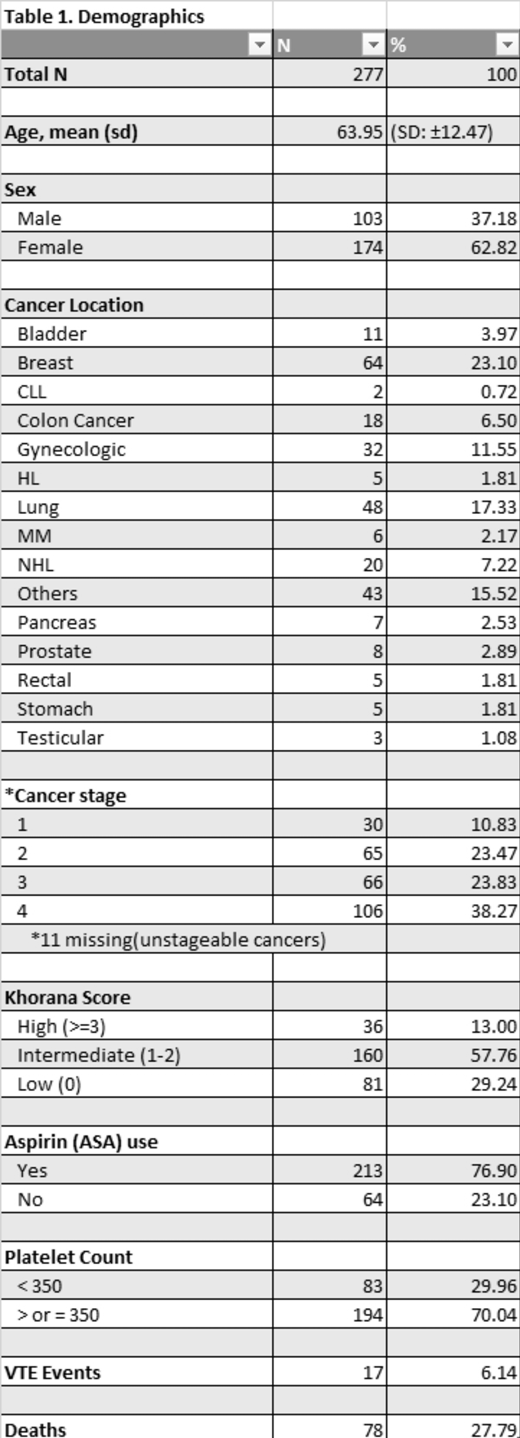
Results: A total of 339 patients were screened and 277 met the eligibility criteria. The mean age of participants was 63.95 (SD ± 12.47). Detailed demographics are presented in Table 1. The incidence proportion (cumulative incidence) of VTE was 6.13%. There were no cases of VTE reported in patients with cancer stage 1 while there were increasing numbers of VTE events with increasing cancer stage (3, 4 and 10 cases were reported with stages 2, 3 and 4 respectively). Patients with metastatic cancer had a greater risk of having a VTE event than those without metastatic cancer though this was not statistically significant (OR= 2.28; 95% CI 0.84 to 6.18; p=0.11). Compared to those with a low KS (0), those with a high KS (≥3) had a significantly greater risk of a VTE (OR, 6.40; 95% CI, 1.17 to 34.58; P=0.03). We observed a modest trend for increased risk of VTE with aspirin use (OR, 2.81; 95% CI, 0.98 to 8.04; P = 0.06) though this was not statistically significant.
An increased risk of death was observed among those patients who had a VTE compared to those who did not have a VTE (OR, 4.03; 95% CI 1.48 to 11.02; P = 0.006). Compared to those with a low KS, those with a high KS had a greater risk of mortality at 6 months (OR, 5.70; 95% Cl 1.32 to 24.66, P=0.02) and up to 2.5 years (OR, 5.04; 95% Cl 1.87 to 13.54, P=0.001) after adjusting for cancer stage. Compared to those with a low KS, those with an intermediate KS (1-2) also had an increased risk of mortality over the 2.5 year study period when adjusted for cancer stage (OR, 2.54; Cl 1.19 to 5.42; p = 0.012) though 6 months mortality was not statistically significant (OR, 2.54; 95% CI 1.19 to 5.42; p = 0.26).
Limitations: This was a retrospective single center study over a small time period. We were limited by the small sample size which may account for the large confidence intervals and lack of precision in the odds ratio estimates. Use of aspirin was not randomized and the effect of specific chemotherapies were not evaluated.
Conclusion: In community hospital setting, high KS was associated with increased odds of VTE. High and intermediate KS were also statistically associated with increased mortality over the 2.5 years study period. We did not observe a protective effect on VTE with aspirin use, instead there was a weak trend to increased risk with its use.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal