Background:
An expert panel convened by the NHLBI in 2014 provided guidelines and indications for blood transfusions in patients with sickle cell disease (SCD). These recommendations were to help conserve the use of blood transfusions in SCD patients in order to reduce the complications of iron overload, transmission of blood-borne pathogens and development of alloantibodies. In a retrospective review of SCD patients who received blood during inpatient admissions we identified deviations from guideline recommendations including transfusions for pain, and asymptomatic drops in hemoglobin(hgb). A standardized education program was subsequently developed and implemented to improve compliance with NHLBI recommendations for transfusion use in hospitalized patients with SCD.
Methods:
A standardized education program was developed based on American Society of Hematology (ASH) and NHLBI recommendations. This program included a PowerPoint presentation which was delivered to small groups of hematologists, hospitalists, emergency room and primary care physicians, nurse practioners and medical residents who regularly manage inpatient SCD admissions. A post-test of knowledge was administered and completed by 65 individuals. Additionally, the ASH pocket guide app was placed on physician's smartphones. Following completion of the program, transfusion use and indications were reassessed over the next 6 months. The hospital database was screened for admissions of patients with the diagnosis of SCD (HbSS, HbSβ0, HbSβ+, HbSC) who were billed for a blood transfusion between 8/1/2015 to 8/31/2016. This served as the baseline data. All patients were >18 years of age. Each chart was reviewed individually to assess the clinical setting and reasons for transfusion. The following parameters were documented: age, sex, length of hospital stay (LOS), hgb at time of transfusion, baseline hgb, number of units transfused, documented reason for transfusion, presence of alloantibodies and ferritin level. We defined the infusion of blood as a transfused event which included single or multiple units of blood. We excluded transfusions given for acute bleeding. Following the educational program, this same methodology was used to collect a second cohort of SCD patients admitted to the hospital between 1/2/2019-6/22/2019 wherein the indications and outcomes of blood transfusion were compared with the first, pre-educational cohort.
Results:
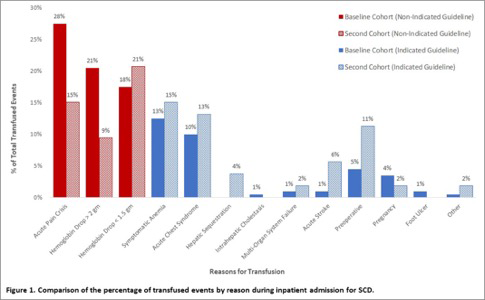
In phase one (pre-education cohort), a total of 87 SCD patients were analyzed and the majority of inpatient transfusions were not indicated by national guidelines with only 34.5% of transfusion events meeting expert panel recommendations. In this phase of our study, we reviewed a second cohort of 53 SCD patients (post-education). A total of 123 units of blood were transfused. 73.5% of patients had HbSS disease, 22.6% had HbSβ0 disease and 3.77% had HbSC disease. The mean age was 32.5 years and 69.8% were female. Compared to management of patients prior to the education program, less blood was administered for the non-guideline indications of acute pain crisis (15% vs 28%) and Hgb drop >2gm (9% vs 21%) (see Figure 1). The mean level of ferritin in the second cohort was 1751 and 7.54% of patients had alloantibodies, compared to the baseline group with ferritin of 2483 and alloantibodies of 17%. The average LOS in the baseline group was 9 days and in the second cohort was 8.4 days with an average LOS for uncomplicated pain or a drop of hemoglobin in the baseline group of 8.9 days and in the second cohort of 6.6 days. The most common indicated guideline reasons for transfusion in both cohorts were for symptomatic anemia and acute chest syndrome.
Conclusion:
This educational program was able to reduce transfusion use in SCD patients who were hospitalized for vaso-occlusive crises and asymptomatic anemia. There was a trend towards decrease in LOS for patients when they had less transfusions. Face to face education in combination with the use of the readily available ASH pocket app on smartphones are effective tools to increase adherence to national guidelines for transfusion in SCD.
Cohen:Global Therapeutics: Other: Trial for GBT440.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal