Introduction: Allogeneic hematopoietic stem cell transplantation (alloHSCT) offers an effective, potentially life saving treatment for a broad range of malignant and nonmalignant hematologic disorders. To some degree, an adequate performance status, comorbidity and disease control, and an available donor must be present in order to be considered as a suitable candidate. In addition, sociodemographic factors such as insurance coverage, high out-of-pocket costs and transplant center availability might impair access to alloHCT, particularly in low and middle-income countries (LMIC). Currently, in the era of haploidentical grafts donor availability has been largely resolved and is no longer the biggest barrier for reaching transplantation in LMIC, becoming the most common form of alloHSCT in our center. On the other hand, most outcomes-based publications in the field of alloHSCT are focused only on the transplanted, not on an "intent-to-transplant" basis. The current reasons for not being transplanted once a patient is considered for the procedure are unknown. Therefore, as early adopters of haploidentical transplantation we aimed to evaluate patients that were not grafted.
Objetive: To determine barriers to alloHSCT and patient outcomes by studying the population that was HLA typed and/or had a consult in our center, regardless of the event of transplantation
Methods: All adult patients who were HLA-typed and considered potentially eligible for transplantation in our center were included. Since 2015 haploidentical donors have become the immediate next best option when a matched sibling donor is unavailable in our center, due to a lack of access and increased costs of unrelated donors and umbilical cord blood. Related alloHSCT have been largely subsidized by the government, reducing transplant costs to a minimum for uninsured patients. We performed a retrospective review of patient records from January 2016- December 2018, regardless of baseline diagnosis, donor source and conditioning regimen. We documented demographic variables, identified the reasons for not performing the alloHCT, and performed a landmark analysis from the time of HLA typing in comparison to patients that did reach transplantation. Univariate and multivariate analyses were performed to identify covariates associated to not reaching transplantation.
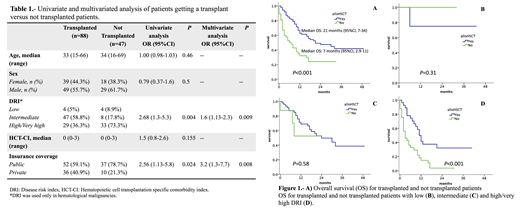
Results: One hundred and thirty five patients were HLA-typed and considered eligible to proceed to alloHCT. Forty-seven (34.8%) were not transplanted. In six cases, alloHSCT was considered unnecessary. The proportion of patients who received transplantation was similar regardless of the availability of a MSD vs haploidentical donor (68.2 vs 66.7% repectively, p=0.53). Transplanted patients had a significantly lower disease risk index (DRI) and more frequently had private healthcare. Other baseline characteristics between transplanted and not transplanted cohorts are presented in Table 1. The reasons for not proceeding with transplantation were disease status (refractory disease or relapse prior to conditioning) in 15 patients (36.6%), followed by procedure rejection by patient/family and/or treatment abandonment in 12 (29.2%), 5 (12.2%) did not have a suitable donor, 4 (9.7%) due to economic difficulties, and 4 (9.7%) developed important comorbidities. A high DRI and lack of insurance coverage were associated with a higher risk for not getting a transplant in both univariate and multivariate analyses (Table 1). As expected, the outcome of patients that were not transplanted was dismal overall and particularly in high/very high DRI patients (Figure 1, Panel A-D).
Conclusion: In the era of haploidentical transplantation, the biggest barrier in our limited-resource context is disease activity/relapse, followed by patient rejection/treatment abandonment, and not the availability of a donor. We must perform transplants promptly and increase education for patients and family in order to overcome these limitations.
Gomez-Almaguer:Amgen: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Teva: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal