Introduction:
The only cure for sickle cell disease (SCD) is allogeneic hematopoietic stem cell transplant (HSCT) although autologous HSCT of genetically engineered hematopoietic stem cells is promising. Lack of suitable matched related donors (MRD) is a major limitation driving interest in improving outcomes using unrelated donors. While excellent outcomes are achieved with non-myeloablative MRD HSCT in adults (Hsieh et al, 2009 and 2014), results from matched unrelated donor (MUD) HSCT have been limited by excessive graft versus host disease (GVHD) and treatment-related mortality (Shenoy et al, 2016). Here we present updated follow up of our institutional experience using MUD and mismatched unrelated donors (MMUD) in comparison to patients with MRD.
Methods:
Eligibility for HSCT included frequent pain crises requiring hospitalization and evidence of end-organ damage. Non-myeloablative conditioning with alemtuzumab and 3 Gy total body irradiation (TBI) was used for MRD HSCT (n=7), whereas patients without an MRD were transplanted using MUD, MMUD or haploidentical grafts (n=6) on a previously reported institutional protocol after conditioning with alemtuzumab (54 mg/m2), fludarabine (180 mg/m2), and melphalan (140 mg/m2), using a CD34+ selected graft with CD3+ cell add back. MRD recipients received sirolimus as GVHD prophylaxis. Non-MRD recipients initially received tacrolimus as GVHD prophylaxis (n=1) but subsequently received sirolimus (n=5) due to the first patient developing posterior reversible encephalopathy syndrome (PRES). All grafts were G-CSF mobilized peripheral blood grafts and all patients underwent RBC exchange to achieve Hgb S <30%. Data is reported using n (%) or median (range) and Wilcoxon rank-sum test was used for continuous variables.
Results:
Median follow up is 21.7 months (range 4.7 - 63.4). Median age for MRD recipients was 28.7 (21.4 - 35.5) years and 22.8 (18.5 - 34.6) for non-MRD recipients. Of note, the MRD group included one patient with a renal allograft from the same donor and another with stage V renal disease awaiting a kidney transplant. All patients where homozygous for hemoglobin S except one who had hemoglobin Sβo -thalassemia in the MRD group, and another heterozygous for hemoglobin S and C in the non-MRD group. Patients in the MRD group received unmanipulated grafts with a median of 14 (6.2 - 16.9) x 10E6 CD34+ cells/kg. Non-MRD recipients received CD34 - selected grafts with a median of 7.8 (4.1 - 15.1) x 10E6 CD34+ cells/kg with 2.2 x 10E5 (0.1 - 2.5) CD3+ cells add back. No growth factors were used post-transplant.
All patient engrafted with no cases of graft failure. Median time to engraftment was significantly longer for the MRD group at 25 (22 - 30) vs 19 (13 - 21) days, p=0.003. Two patients in the MRD group developed acute/late acute GVHD (2 grade II), and 3 patients in the non-MRD group (1 grade II, 2 grade III), 2 of which developed in while switching immune suppression due to PRES. All GVHD cases were steroid responsive and resolved. Three patients in the non-MRD group developed PRES and none in the MRD group. There were no cases of treatment related mortality and all patients are alive and free of SCD.
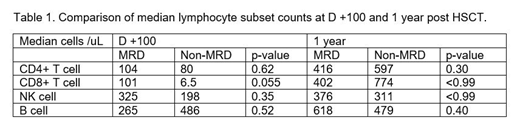
As both groups received alemtuzumab, and the non-MRD group received a CD34-selected graft, we examined lymphocyte subset reconstitution at day 100 and 1 year post-HSCT. The most striking difference was in median CD8+ T cell counts at day +100 which were lower in the non-MRD group approaching significance [101 (43 - 2995) vs 6.5 (3 - 2233) cells/uL, p=0.055, for the MRD and non-MRD respectively]. CD8+ T-cell counts were not significantly different at 1 year [402 (184 - 1066) vs 774 (143 - 1002) cells/uL, p<0.99]. Results from other lymphocyte subsets including CD4+ T-cells, NK cells and B cells are shown in table 1 and were not significantly different between the 2 groups. Of note, early donor T-cell chimerism at D100 was not significantly different between MRD and non-MRD groups [27.0 (18.0 - 50.0) % vs 37.5 (3.0 - 80.0) %, p=0.83] whereas at 1-year, MRD group donor T cell chimerism was significantly lower [53.5 (17.0 - 65.0) % vs 82.7 (69 - 90), p=0.01].
Conclusion:
We demonstrate excellent outcomes with 100% survival and no graft rejection following matched and mismatched unrelated donor HSCT for adult patients with severe SCD. Larger cohorts are needed to confirm these results and further delineate the impact of T-cell subset reconstitution on early-post transplant complications.
Assal:Incyte corporation: Consultancy, Research Funding; boston biomedical: Consultancy. Bhatia:BMS: Consultancy; Vertex Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Bhutani:Sanofi: Membership on an entity's Board of Directors or advisory committees. Lentzsch:Abbvie: Consultancy; Clinical Care Options: Speakers Bureau; Sanofi: Consultancy, Research Funding; Multiple Myeloma Research Foundation: Honoraria; International Myeloma Foundation: Honoraria; Karyopharm: Research Funding; Columbia University: Patents & Royalties: 11-1F4mAb as anti-amyloid strategy; Caelum Biosciences: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy; Janssen: Consultancy; Takeda: Consultancy; BMS: Consultancy; Proclara: Consultancy. Reshef:Magenta: Consultancy; Kite: Consultancy, Research Funding; Atara: Consultancy, Research Funding; Pfizer: Consultancy; BMS: Consultancy; Pharmacyclics: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Celgene: Research Funding; Shire: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal