HSCT is the only curative option for many malignant and non-malignant diseases. The WBMT was founded as an umbrella organization of societies involved in cellular therapies with the mission of promoting excellence in HSCT. As a non-governmental organization in official relation with the World Health Organization (WHO), the WBMT assisted in the founding of regional societies (Latin America Blood and Marrow Transplantation Group and African Blood and Marrow Transplantation Group), performs global activity surveys, conducts workshops and provides expert support for programs in evolving countries. In this retrospective evaluation we analyzed worldwide activity trends in HSCT up to the year 2016 and evaluated possibilities of improving availability of HSCT by the use of telemedicine.
Methods: HSCT activity was collected annually from member societies, national registries and individual centers including donor type (allogeneic/autologous), stem cell source (bone marrow/peripheral blood stem cells/cord blood) and indications for transplant. Transplant rates (TR) were calculated as HSCT/10 million inhabitants without adjustment for patients transplanted in a country other than that of primary residence. Country team density (TD) was defined as teams/10 million inhabitants. Workshops were organized in a number of locations where there was little or no HSCT activity or where improvement in one or more aspect of local or regional HSCT activity was requested, including Vietnam, Brazil, China, South Africa and Morocco. Other countries were paired with established centers using WBMT affiliated partners. In two countries, a pilot program was established involving a 6 month physician training in a JACIE/FACT accredited center followed by daily telemedicine-guided supervision of clinical activities.
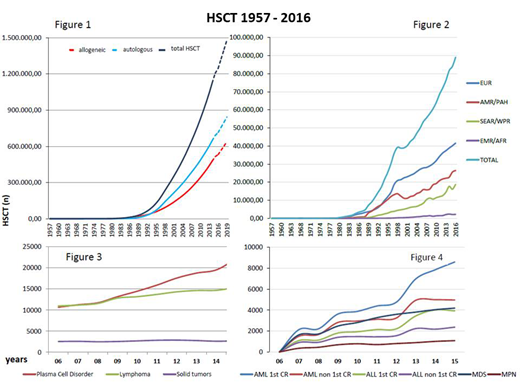
Results: From 1957-2016 a total of 1,298,897 HSCT (57.1% autologous) procedures were collected. By the end of 2016, HSCT activity was reported from 87 of the 195 WHO member states. A total of 89,070 HSCT from 1662 centers was reported in 2016. Assuming a frequency of 84,000/year, 1.5 million HSCT will be reached in 2019, only 7 years after the 1 million report in 2012 (Figure 1). The global activity/year increased continuously from 10,000/year in 1991 to 82,718 first HSCT/year in 2016 with a global increase of >7% (7.0% in autologous and 7.8% in allogeneic HSCT). As in previous years, slightly more autologous (53.5%) than allogeneic and more related (53,6%) than unrelated HSCT were reported. The further increase in related HSCT was caused mainly by an increase of non-identical family donors (39.5% of related HSCT). Increase in activities according to regions is given in Figure 2. TR and TD varied according to region and are highest in Nord America with 511.2 TR, followed by Europe with 390.9 TR, Latin America with 63.9 TR, APBMT with 46.2 TR and Africa/EMRO with 32.8 TR. In contrast, TD was highest in Europe (7.5 TD) followed by Nord America (6.0 TD), APBMT (1.9 TD), Latin America (1.9 TD) and Africa/EMRO (0.4 TD). Commonest indications were lymphoproliferative diseases for autologous and leukemia for allogeneic HSCT and continue to rise (Figure 3 autologous and Figure 4 allogeneic HSCT). Graft source were predominantly peripheral blood in autologous (99.7%) and 65% in unrelated HSCT, while umbilical cord blood as a stem cell source (13.8% of all unrelated) declined. More than 150 HSCT were performed in one country and one center without activities using daily telemedicine-guided supervision.
In conclusion, the global distribution and activities are increasing continuously by more than 7,0% per year with numbers currently running at app. 90,000/year. Of note is the increase of haploidentical HSCT activity, while the use of umbilical cord blood HSCT continues to decrease. TR data show significant gaps between regions. Supervisory telemedicine is a powerful tool to overcome lack of experience and establish JACIE/FACT compatible new programs with collateral benefits for conventional hematology, blood banking, microbiology and virology.
Abbreviations: EUR, Europe; AMR/PHA, America; SEAR/WPR, South-East Asia/Western Pacific; AFR/EMRO, African/Eastern Mediterranean.
Niederwieser:Daichii: Speakers Bureau; Cellectis: Consultancy. Atsuta:Kyowa Kirin Co., Ltd: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Mochida Pharmaceutical Co. Ltd: Honoraria; Janssen Paharmaceutical K.K.: Honoraria. Worel:Sanofi Genzyme, Malinckrodt Therakos: Research Funding; Jazz, Sanofi, Celgene, Novartis, Malinckrodt Therakos: Honoraria; Sanofi Genzyme, Malinckrodt Therakos: Speakers Bureau. Galeano:Szabo SA: Other: (Equity interest). Novitzky:Astellas, Roche: Consultancy. Szer:Prevail Therapeutics: Honoraria, Other: Travel, Research Funding; Novartis: Honoraria, Other: Travel, Research Funding; MSD: Honoraria, Other: Travel, Research Funding; Celgene: Honoraria, Other: Travel, Research Funding; Amgen: Honoraria, Other: Travel, Research Funding; Alexion: Honoraria, Other: Travel, Research Funding; Sanofi: Honoraria, Other: Travel, Research Funding; Takeda: Honoraria, Other: Travel, Research Funding; Pfizer: Honoraria, Other: Travel, Research Funding. Kröger:Celgene: Honoraria, Research Funding; DKMS: Research Funding; JAZZ: Honoraria; Medac: Honoraria; Neovii: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Riemser: Research Funding; Sanofi-Aventis: Research Funding. Weisdorf:Incyte: Research Funding; Pharmacyclics: Consultancy; Fate Therapeutics: Consultancy. Pasquini:Amgen: Consultancy; BMS: Research Funding; Medigene: Consultancy; Pfizer: Other: Advisory Board; Novartis: Research Funding; Kit Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal