Background: Multiple myeloma (MM) remains incurable with eventual relapse and death occurring despite multiple lines of chemotherapy. Standard frontline therapy for MM has traditionally consisted of combinations of immunomodulatory drugs (IMiDs), proteasome inhibitors (PIs), and dexamethasone followed by consolidation with high-dose melphalan and autologous stem cell transplantation (ASCT). A randomized trial using IMiD and PI-based induction demonstrated significant progression-free survival (PFS) benefit with upfront ASCT in consolidation compared to ASCT in second-line at relapse, but no OS benefit (Attal et al, NEJM, 2017). Delay of ASCT to second-line or beyond may in part be due to the aforementioned lack of OS benefit shown with upfront ASCT and increase of sustained deep responses, including MRD negativity, to novel frontline 3 and 4-drug regimens and continuation of therapy in the frontline. We performed a chart review comparing PFS1, PFS2, and overall survival (OS) of patients who underwent upfront ASCT as consolidation to those who received ASCT in the second-line after one relapse ('delayed ASCT'). The study was approved by the Institutional Review Board at Weill Cornell Medical College.
Methods: 124 MM patients who underwent ASCT either as consolidation in first-line or delayed ASCT after relapsing on one prior treatment between 2010-2016 were included. Demographics and clinical parameters were extracted from the electronic medical record. PFS1, PFS2, and OS were calculated from date of diagnosis to first relapse, second relapse, and death, respectively. Patients were censored if lost to follow-up prior to experiencing the relevant event. PFS1, PFS2, and OS of patients receiving upfront ASCT were compared to those of patients receiving delayed ASCT. Log-rank tests were used to statistically evaluate differences between Kaplan-Meier PFS/OS curves. Cox proportional hazards models were used to calculate hazard ratios (HR) using upfront ASCT as the reference treatment.
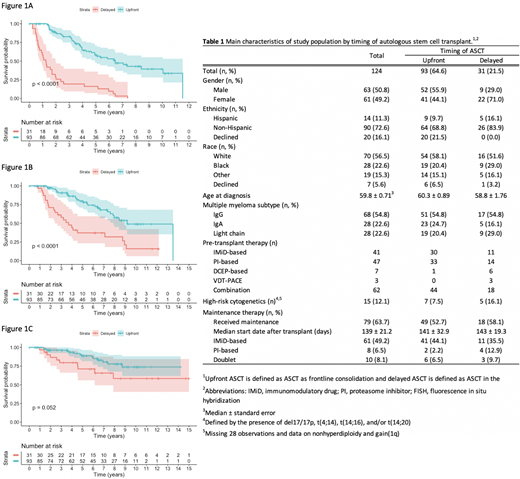
Results: Among the 124 patients, 93 underwent upfront ASCT as consolidation and 31 underwent delayed ASCT after relapsing on one prior line of therapy (Table 1). Induction regimens and pre-transplant therapies received are detailed in Table 1. Of the delayed group patients, 6 underwent ASCT directly without second-line induction and 25 as consolidation after second-line therapy. Patients receiving upfront ASCT had significantly longer median PFS1 compared to patients who received delayed ASCT (6.45 vs 1.25 years; P<0.001), with a HR of 0.18 (95% CI, 0.11-0.29) (Figure 1A). Median PFS2 in the upfront ASCT group was likewise significantly longer than in the delayed group (9.19 vs 3.69 years; P<0.001), with a HR of 0.31 (95% CI, 0.18-0.55) (Figure 1B). With a median follow-up of 6.0 and 5.8 years in the upfront and delayed groups, respectively, median OS was not reached in either group but trended towards prolonged survival with upfront ASCT (P=0.052), with a HR of 0.46 (95% CI, 0.20 to 1.03) (Figure 1C).
Conclusions: In this cohort of 124 MM patients undergoing ASCT as consolidation in the upfront setting or delayed ASCT in second-line after relapse, upfront ASCT was associated with significantly improved PFS1 and PFS2, similar to findings by Attal et al. However, in that study, no difference in OS was seen between upfront and delayed groups, while our data, with longer follow-up, showed median OS trending towards significance. This has important implications as with use of novel induction regimens and maintenance therapy, ASCT is more commonly being delayed to early relapse in second-line or beyond. If transplant is intended to be used in the first few lines of therapy, our data show that delaying ASCT even to second-line has a significant negative impact on PFS. In addition, a shorter OS with delayed transplant was suggested although did not reach statistical significance, possibly due to small numbers or more patients with high-risk MM in the delayed group. It is also important to note the lack of daratumumab-based regimens which may have improved PFS/OS in either or both arms. Thus, our findings should be prospectively validated in a larger trial of ASCT as consolidation in first-line vs second-line or beyond using novel, monoclonal antibody-based regimens.
Rosenbaum:Janssen: Research Funding; Honoraria Akcea: Other: Accordant Health. Coleman:Gilead, Bayer, Celgene: Consultancy, Research Funding, Speakers Bureau; Kite Pharmaceuticals: Equity Ownership; Merck: Research Funding; Pharmacyclics: Speakers Bureau. Van Besien:Miltenyi Biotec: Research Funding. Niesvizky:Takeda, Amgen, BMS, Janssen, Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal