Background: Autologous stem cell transplantation (ASCT) is a curative therapy for a significant proportion of patients with relapsed/refractory (R/R) classic Hodgkin lymphoma (cHL). We previously developed a conditioning regimen for R/R cHL patients undergoing ASCT incorporating gemcitabine and vinorelbine (GN-BVC), which enabled a reduction in the BCNU dose and toxicity without compromising efficacy (BBMT 2010;16:1145-54). Since this publication, several novel agents have been approved for treatment of R/R cHL including brentuximab vedotin (BV) and the immune checkpoint inhibitors (ICIs), which may be changing outcomes and toxicities following ASCT. We sought to determine whether post-ASCT outcomes have improved for R/R cHL in the era of novel agents, and to evaluate how BV and the ICIs have impacted toxicities after ASCT in a large cohort of patients treated with the same conditioning regimen.
Methods: We conducted a single-center, retrospective analysis of outcomes for a large cohort of patients with R/R cHL who underwent ASCT using GN-BVC conditioning at Stanford University from 2001-2017 (n=268). We divided the cohort into two treatment eras: Era A: 2001-2009 (n=137) and Era B (increasing use of novel agents): 2010-2017 (n=131). We used Kaplan-Meier and Cox models to compare overall survival (OS), progression-free survival (PFS), and post-progression survival (PPS) between treatment eras. We also compared outcomes between patients who underwent ASCT in complete remission (CR, n=126) or with a partial response (PR, n=142). For patients treated in era B, we compared outcomes and toxicity profiles for patients treated with standard platinum-based salvage regimens (n=102) to those who received BV-based salvage regimens prior to ASCT (n=29).
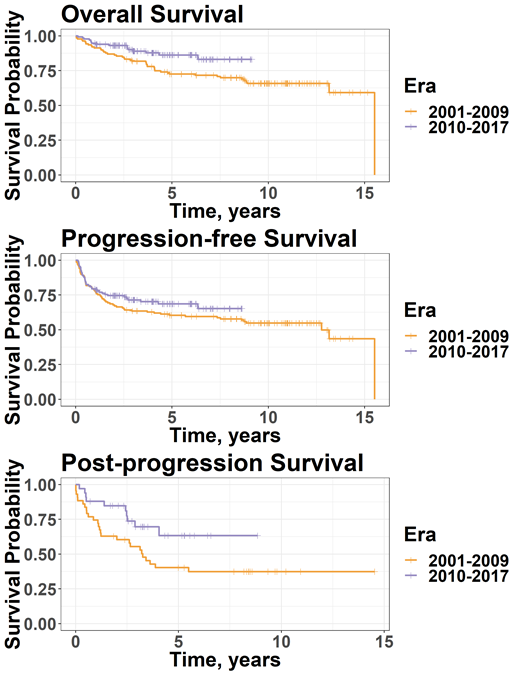
Results: Patients who underwent ASCT in era B had improved OS compared to those transplanted in era A (4-year OS: 87.8% vs 77.9%, HR 0.51, 95% CI 0.28-0.91, p=0.022), but PFS did not differ significantly between treatment eras (4-year PFS: 70.2% vs 62.7%, HR 0.78, 95% CI 0.52-1.2, p=0.24, Figure 1). For patients who relapsed after ASCT, PPS was significantly higher for those transplanted in era B compared to era A (84.7% vs 62.8% at 2 years, HR 0.47, 95% CI 0.23-0.98, p=0.045). Overall, patients who underwent ASCT in CR compared to those with a PR had significantly improved OS (88.3% vs 78.3% at 4 years, HR 0.50, 95% CI 0.29-0.88, p=0.016) and PFS (78.4% vs 55.1% at 4 years, HR 0.44, 95% CI 0.28-0.67, p=0.00016). Among patients who relapsed, those in era B who received novel agents (BV and/or an ICI) at any point in treatment exhibited higher OS compared to historical controls in era A (4-year OS: 72.2% vs 53.1%) but were statistically different only at the p=0.083 level which is above the p=0.05 significance threshold.
Of the patients who underwent ASCT in era B (n=131), 102 (78%) received standard platinum-based salvage regimens (e.g. ICE or DHAP) and 29 (22%) received BV-based salvage regimens prior to ASCT, including 11 patients (8%) who received both BV and an ICI pre-ASCT. Outcomes were excellent for patients who received BV-based salvage regimens pre-ASCT (4-year OS 97% and PFS 76%) but were not statistically different compared to patients who received platinum-based regimens (4-year OS 86% and PFS 69%; p=0.56 and p=0.76, respectively). Pneumonitis requiring corticosteroids occurred in 8% of patients treated in era B, including 10% of patients receiving BV-based salvage regimens pre-ASCT and 7% of patients receiving standard platinum-based regimens. One patient who received BV and nivolumab pre-ASCT died from grade 5 pneumonitis.
Conclusions: In this cohort of R/R cHL patients who underwent ASCT using the same conditioning regimen, OS was significantly higher for patients transplanted within the past decade. A potential survival benefit was observed among patients who relapsed post-ASCT, likely reflecting the more widespread use of BV and ICIs in the post-ASCT setting. Patients who received BV-based salvage regimens pre-ASCT had excellent outcomes without an apparent increase in toxicity, but the small number of patients limits comparisons to standard platinum-based regimens. Further studies are needed to better define the optimal sequencing of novel agents and ASCT in the treatment of R/R cHL in the modern era.
Sica:Physician's Education Resources (PER): Honoraria. Advani:Celgene: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cell Medica, Ltd: Consultancy; Kyowa Kirin Pharmaceutical Developments, Inc.: Consultancy; Regeneron: Research Funding; Celmed: Consultancy, Membership on an entity's Board of Directors or advisory committees; Stanford University: Employment, Equity Ownership; Seattle Genetics: Consultancy, Research Funding; Agensys: Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Autolus: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kura: Research Funding; Merck: Research Funding; Infinity Pharma: Research Funding; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead Sciences, Inc./Kite Pharma, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Forty-Seven: Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium: Research Funding. Miklos:AlloGene: Membership on an entity's Board of Directors or advisory committees; Precision Bioscience: Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotech: Membership on an entity's Board of Directors or advisory committees; Becton Dickinson: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Kite-Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Muffly:Pfizer: Consultancy; Adaptive: Research Funding; KITE: Consultancy. Rezvani:Kaleido: Membership on an entity's Board of Directors or advisory committees, Other: one-time compensation from advisory boards; Nohla Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: one-time compensation from advisory boards; AbbVie: Other: Principal investigator ; U.S. Department of Justice: Other: Expert medical witness; Johnson & Johnson: Employment, Other: Brother is employed. Shizuru:Forty Seven Inc: Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal