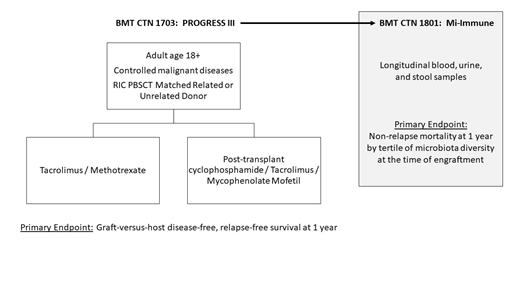
Graft-versus-host disease (GVHD) remains the major source of morbidity and transplant-related mortality after allogeneic hematopoietic cell transplant (alloHCT). Since the mid-1980s, the standard prophylaxis for GVHD has been a calcineurin inhibitor plus methotrexate (MTX). Subsequent clinical trials designed to intensify GVHD prophylaxis have shown an increased risk of relapse, thus negating survival benefit. The ideal GVHD prophylaxis regimen is therefore associated with a high graft-versus-host disease-free, relapse-free survival (GRFS), a composite endpoint that reflects survival without morbidity. A recent 3-arm randomized phase II study conducted through the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 1203) showed a benefit in GRFS using post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil (PTCy/Tac/MMF) with a hazard ratio of 0.72 (90% confidence interval 0.54 - 0.94, p-0.044) compared to contemporary non-randomized CIBMTR Tac/MTX controls. Based upon these results, the BMT CTN has launched 1703, a multicenter randomized phase III study of standard Tac/MTX vs PTCy/Tac/MMF in the setting of reduced intensity conditioning, matched peripheral blood stem cell transplantation (RIC PBSCT). The primary endpoint of 1703 is GRFS at 1 year, and several secondary endpoints, including patient-reported outcomes, will be analyzed. A series of recent, predominantly single-center studies have shown an association with the composition of intestinal microbiota and the development of acute GVHD, which suggests that this may be a potential modifiable biomarker of disease. The generalizability of findings from these studies is yet unknown, and no large multi-center studies on the intestinal microbiota of HCT patients has yet been carried out. Patients enrolled in 1703 will be eligible to co-enroll in BMT CTN 1801, the Mi-Immune study, detailing microbiota and immune reconstitution in this cohort. The primary endpoint of 1801 is 1-year non-relapse mortality by tertiles of stool microbial diversity at engraftment. Secondary objectives, including effect of GVHD prophylaxis on diversity, oligodomination and risk of bloodstream infection, volume of antimicrobial exposure, and T-cell receptor diversity, will be analyzed. The combined studies of BMT CTN 1703/1801 will define the standard of care for GVHD prophylaxis in the RIC PBSCT setting and vastly expand our knowledge on the impact of intestinal microbiota in critical outcomes after alloHCT. This clinical trial is registered at ClinicalTrials.gov NCT03959241.
Holtan:Incyte: Consultancy; Bristol-Myers Squibb: Consultancy; Janssen: Consultancy; CSL Behring: Consultancy. Bhatt:ArcBio: Other: Scientific Advisory Board; January.ai: Other: Scientific Advisory Board; Caribou Bioscience: Other: Scientific Advisory Board; Kaleido Biosciences: Consultancy, Other: Paid Consultant; Janssen Human Microbiome Institute: Consultancy, Other: Paid Consultant; Illumina: Honoraria; 10x Genomics: Other: Research collaboration without funding support; Illumina: Other: Research collaboration without funding support; Agilent: Research Funding; Global Oncology, Inc: Other: Nonprofit Board. Perales:Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; MolMed: Membership on an entity's Board of Directors or advisory committees; NexImmune: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria; Medigene: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Kyte/Gilead: Research Funding; Miltenyi: Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kean:HiFiBio: Consultancy; BlueBirdBio: Research Funding; Gilead: Research Funding; Regeneron: Research Funding; EMDSerono: Consultancy; FortySeven: Consultancy; Magenta: Research Funding; Kymab: Consultancy; Jazz: Research Funding; Bristol Meyers Squibb: Patents & Royalties, Research Funding. Hamadani:Medimmune: Consultancy, Research Funding; Takeda: Research Funding; Pharmacyclics: Consultancy; Janssen: Consultancy; Merck: Research Funding; Celgene: Consultancy; Otsuka: Research Funding; ADC Therapeutics: Consultancy, Research Funding; Sanofi Genzyme: Research Funding, Speakers Bureau. Bolaños-Meade:Incyte Corporation: Other: DSMB fees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal