Introduction
Transplant associated thrombotic microangiopathy (TA-TMA) is a life-threatening complication post hematopoietic stem cell transplant (HSCT). Epidemiology, risk factors, prognosis and treatment of TA-TMA are not very clear. While type of transplant, and use of calcineurin inhibitors (CNIs) are established risk factors for TA-TMA, not much is known of other risk factors that affect TA-TMA and its outcome. We retrospectively analysed data of all patients who underwent allogeneic transplant for hematological malignancies at our centre to understand its incidence, possible risk factors and treatment outcomes.
Methods
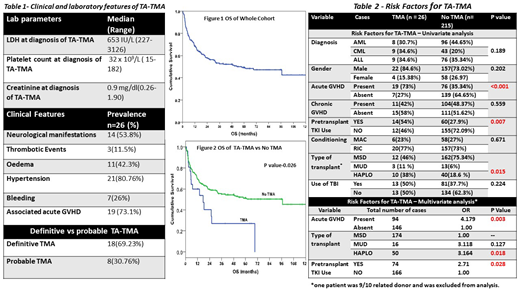
All consecutive patients who underwent allogeneic HSCT for AML, ALL and CML from January 2008 to March 2019 were included. Conditioning regimens used were either myeloablative (CyTBI or BuCY) or reduced intensity (fludarabine with either melphalan, treosulfan or cyclophosphamide with or without 2 Gy TBI). Rabbit ATG was used at a dose of 2.5-5 mg/kg for patients who underwent matched unrelated donor (MUD) transplants. GVHD prophylaxis for matched sibling donor (MSD) and MUD transplants included CNIs with methotrexate or mycophenolate mofetil (MMF). Patients who underwent haploidentical (haplo) HSCT received post transplant cyclophosphamide (PTCy) on d+3 and d+4 and CNI with MMF from day+5 onwards. Definitive TA-TMA was defined using Blood and Marrow Transplant Clinical Trials Network (BMT-CTN) criteria ie schistocytes ≥2/HPF, elevated LDH, renal compromise (increase in creatinine 2 times baseline or 50 % decrease in creatinine clearance from baseline ) or unexplained neurological manifestations and negative coombs test. Probable TMA was defined as per criteria proposed by Cho et al ie schistocytes ≥2/HPF, elevated LDH, negative coombs test, no coagulopathy, drop in haemoglobin and thrombocytopenia (platelet < 50 x 109/L or > 50 percent decline in platelet count). Risk factors explored for development of TA-TMA were age, gender, diagnosis, type of transplant, acute and chronic GVHD, use of tyrosine kinase inhibitors (TKI) such as imatinib, dasatinib, nilotinib or sorafenib pre transplant and conditioning regimen used. Factors evaluated to affect the outcomes of TA- TMA were gender, baseline LDH, least platelet count, presence of acute GVHD, TA-TMA index (LDHx1000/platelet count per cumm), definitive vs probable TMA, and type of transplant. All categorical data was compared using Chi-square /Fisher exact test. OS probabilities were calculated using Kaplan -Meier method and were compared using log-rank test. Multivariate analysis to assess risk factors for TMA was carried out using logistic regression.
Results
Total 241 patients, 179 (74.2 %) male and 62 (25.7 %) female, with median age of 28.5 (3- 53) years were analysed. Donors were MSD (6/6 matched -174, and 9/10 matched-1), MUD (16) or haplo (50) donors. Diagnoses were 104(43 %) - AML, 85 (35%) - ALL (of which Ph+ patients --23) and 52 (22%) -CML patients. Conditioning regimens given were myeloablative (MAC; n= 64, 27 %) or reduced intensity (RIC; n=177, 73 %).
Total 26 (10.7%) patients developed TA-TMA; 22 (85%) were males. Table 1 shows clinical features at diagnosis of TA-TMA. Median day of diagnosis of TA-TMA was d+102 (range 12- 722) post HSCT. Analysis of potential risk factors for TA-TMA is shown in table 2. Use of pre-HSCT TKI (OR 2.7, p=0.028), haplo HSCT (OR 3.16, p=0.018) and presence of acute GVHD within 30 days of diagnosis of TA-TMA (OR 4.17, p=0.003) were identified to be significant factors on multivariate analysis. CNIs were omitted as soon as TA-TMA was diagnosed. Low dose defibrotide (5mg/kg BD) was used in 4 patients. No patient received plasma exchange. Median follow up for whole cohort was 59.5 months. Median OS for whole cohort was 59.9 months (figure 1). The OS (figure 2) at 5 years was 51.1% in the patients who did not develop TA-TMA vs 29.6% in the group with TA-TMA (P=0.026).Ten (39%) patients with TA-TMA are alive at the time of analysis. No significant factors affecting outcomes of TA-TMA were identified.
Conclusion
Incidence of TA-TMA was 10% in our cohort. Pre-transplant TKI use, acute GVHD, and haplo HSCT are independent risk factor for TA-TMA. This is the first study in our knowledge to show pre-transplant TKI as a risk factor for development of TA-TMA. Higher index of suspicion of TA-TMA is thus warranted for those patients who receive pre transplant TKI.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal