Background: Prolonged thrombocytopenia after hematopoietic stem cell transplant is a relatively common complication associated with intricate mechanisms including impaired thrombopoiesis and increased platelet turnover. Platelet recovery is particularly delayed after umbilical cord blood transplant (UCBT). Romiplostim is a thrombopoietin receptor agonist that is FDA approved for the treatment of chronic ITP. Although an increasing number of studies recently show promising results on the use of romiplostim in chemotherapy induced thrombocytopenia, the effect of romiplostim on platelet recovery in patients with persistent thrombocytopenia after UCBT remains unknown.
Objectives: The primary objective of the study was to determine the maximum tolerated dose (MTD) of romiplostim in patients who failed to achieve platelet recovery by day +28 after UCBT. Secondary objectives were to determine if romiplostim influences the speed of platelet recovery, decreases the risk of thrombocytopenia related complications or affects the incidence of bone marrow (BM) fibrosis or relapse.
Methods:This was a single center dose escalation trial of weekly romiplostim in patients >18 years who failed to achieve an untransfused platelet count (PLT) of 20 x 109/L by day +28 after myeloablative (MA) or nonmyeloablative (NMA) UCBT. A total of 21 patients were enrolled from April 2015 to December 2018. One patient withdrew and was replaced so 20 patients are included in the analysis. Romiplostim was administered at the assigned dose as 6 weekly injections beginning by day +42 post UCBT with an end date by day +100. Four dose levels (4, 6, 8, and 10 mcg/kg/dose) were evaluated. There was no intra-patient dose escalation. The MTD of romiplostim was determined by the Continual Reassessment Method, with a goal to identify a dose level which corresponds to the desired toxicity rate of £ 20%. Toxicities of interest included thrombosis, PLT>400x109/L and any grade 4-5 adverse event (AE) attributed to romiplostim. The comparison group were historical controls selected from our prospectively collected database based on age, gender, underlying disease, conditioning intensity and transplant type (1:1 matching).
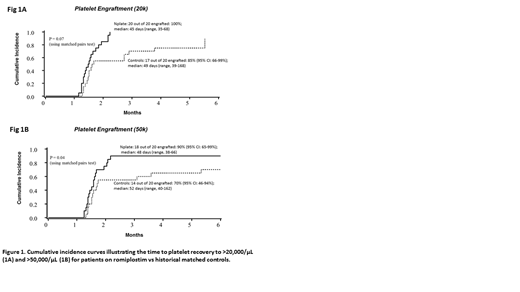
Results: Median (range) age of the patients was 59.5 (18-68) years and 60% were female. Ten patients had AML, 5 ALL, 3 MDS, 1 NHL and 1 MM. Of those, 11 received NMA double UCBT, 7 MA single UCBT and 2 received NMA single UCBT. Two patients received 4 mcg/kg/dose, two 6 mcg/kg/dose, four 8 mcg/kg/dose and the remaining 12 received 10 mcg/kg/dose. Only 5 patients completed the full 6 doses of treatment. Of the 15 patients who received less than 6 doses, 12 were due to a PLT>100×109/L, 2 due to PLT>400×109/L, and 1 was due to physician choice after the subject developed right upper extremity edema (without thrombosis). As shown in figure 1, 100% of romiplostim treated patients achieved platelet engraftment to 20 x 10^9/L at a median of 45 days post UCBT compared to 85% of controls who achieved platelet engraftment at a median of 49 days (p=0.07). Similarly, 90% of romiplostim treated patients achieved platelet engraftment to 50 x 10^9/L at a median of 48 days compared to 70% of historical controls who achieved platelet engraftment at a median of 52 days (p=0.04).
The most common AEs were insomnia (30%), arthralgia (25%), myalgia (20%), headache (20%), dizziness and abdominal pain (15%). Only three patients experienced serious AEs requiring discontinuation of the drug: two had a PLT>400x109 /L (without complications) and one developed right upper extremity edema. Bleeding episodes improved with the use of the drug as well as need of transfusions (patients vs controls, p<0.05). All dose levels appear to be effective with overall low toxicity. Therefore, the MTD of romiplostim was 10mcg/kg/dose.
All study patients were evaluated for BM fibrosis and relapse at regular intervals. There were no BM findings suggestive of increased fibrosis or relapse by day +100, and peripheral blood morphology was normal (no significant dacryocytosis, normal platelet morphology).
Conclusion:Our study showed that romiplostim was well tolerated and accelerates platelet engraftment in patients undergoing UCBT, with the MTD being 10mcg/kg/dose. These results indicate that romiplostim is safe and potentially effective therapy to improve platelet recovery after UCBT. Further studies in larger numbers of patients are needed to confirm these observations.
Wagner:BlueRock: Research Funding; Rocket Pharmaceuticals: Consultancy; Novartis: Research Funding; Magenta: Consultancy, Research Funding; Gadeta: Membership on an entity's Board of Directors or advisory committees. Brunstein:Gamida: Research Funding; Magenta: Research Funding; Astex: Research Funding. Bejanyan:Kiadis Pharma: Other: advisory board. Smith:Amgen: Research Funding; Jazz Pharmaceuticals: Research Funding.
Romiplostim (Nplate) is an FDA approved drug for chronic ITP. In this study (phase I trial) we aimed to evaluate the safety and effectiveness of romiplostim on platelet recovery following UCBT.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal