Cancer chemotherapy may be associated with a high incidence of nausea and vomiting (CINV), which may occur acutely within 24 hours after the start of chemotherapy (acute phase) or in the following days (delayed phase). Despite the availability of several antiemetics, clinical findings show that control of nausea and vomiting continue to be a serious concern for hematological patients, mainly for those receiving multiple-day (MD) and high-dose (HD) chemotherapy (CT), for which no specific international recommendations have been formulated, due to the lack of a unanimous consensus between the main international guidelines. NEPA is the first antiemetic developed as an oral fixed dose combination of two drugs that are antagonists of two receptors involved in the control of nausea and vomiting: a new highly selective NK1-RA, netupitant, and a second generation 5HT3-RA, palonosetron, that simplify the antiemetic regimen allowing for a lower number of capsules and days of treatment. In clinical practice, NEPA is administered together with dexamethasone that contributes to CINV prophylaxis by its intrinsic antiemetic properties. However, dexamethasone also exhibits an important immunosuppressive activity, which could lead to several adverse events, such as increasing the risk of serious infections, especially in patients undergoing myeloablative treatment.
The rational of this study was to explore the efficacy of multiple doses of NEPA given with an every-other-day regimen without dexamethasone in preventing CINV in patients with non-Hodgkin's lymphoma (NHL) eligible for autologous stem cell transplantation (ASCT) and treated with MD-HD-CT. The chemotherapy regimen (BEAM/FEAM) was administered for 6 days, NEPA was taken on day 1, 3 and 5, and nausea and vomiting were monitored up to day 15. No dexamethasone was given for antiemetic prophylaxis. The primary endpoint was the percentage of patients achieving a Complete Response (CR; no vomiting and no use of rescue medication) during the overall phase, defined as the period from day 1 (first day of chemotherapy) until 2 days after the last dose of chemotherapy.
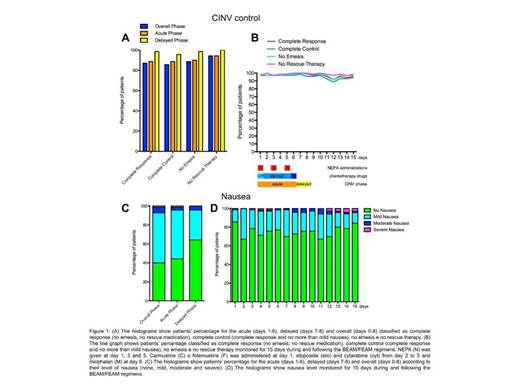
Seventy patients participated to the study. According to the adopted Fleming one-stage design, the primary endpoint of this study was achieved. Indeed, the number of complete responders for the overall phase was 60, which is greater than the predetermined cut-off of 42, representing the minimum frequency of responders for which the treatment is considered effective. In addition to the primary efficacy result, several additional endpoints were evaluated for the study period (Figure 1). The CR values were 87.1% (primary endpoint, overall phase: days 1-8), 88.6% (acute phase: days 1-6) and 98.6% (delayed phase: days 7-8), while the complete control (CR with no more than mild nausea) was 85.7% (overall phase), 88.6% (acute phase) and 95.7% (delayed phase) (Figure 1A). Moreover, the percentages of patients that did not have any emetic episodes were 88.6% (overall phase), 90% (acute phase) and 98.6% (delayed phase) and patients that did not require a rescue therapy for controlling CINV were 94.3% (overall phase), 94.3% (acute phase) and 100% (delayed phase). Daily records taken from day 1 to day 15 showed that values for all these categories were above 85% for all the days of observation (Figure 1B). Patients also documented the grade of their nausea according to the Likert scale. Moderate and severe episodes of nausea were reported by less than 10% for the overall phase and less than 5% in both acute and delayed phase and the daily values of no nausea were above 65% for each day of the treatment (Figure 1C and 1D). Indeed, the mean patient global satisfaction for the antiemetic prophylaxis for the study period was 9.13 ± 1.59 out of 10. Regarding safety, a total of 12 Treatment-Emergent Adverse Events (TEAEs) occurred in 6 (8.6%) subjects enrolled in the study. Among these, only 1 (8.3%, constipation) was evaluated as possibly related to NEPA administration. Moreover, the only two TEAEs classified as SAE (Serious Adverse Event) were two episodes of fever that have been evaluated as not-related to NEPA. Therefore, the safety profile of NEPA was confirmed also in this setting.
In conclusion, our study demonstrated that multiple alternate dosing of NEPA without the addition of dexamethasone can effectively prevent nausea and vomiting in a difficult setting, such as MD/HD-CT with a good tolerability profile.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal