Increasing the numbers of donor hematopoietic stem cells (HSCs) would accelerate hematopoietic recovery in many HSC transplant recipients, thereby reducing mortality, morbidity and costs. HSC gene therapies would further benefit from high transplant donor HSC numbers, due to inefficiencies in genetic modification of HSCs and losses during transfection protocols. Unfortunately, worldwide attempts to optimize culture parameters, such as hematopoietic growth factor combinations, feeder cells and bioengineered chambers, have failed to result in the substantial HSC self-renewal needed clinically, although it was reported recently that substitution of polyvinyl alcohol for albumin resulted in massive expansion of mouse HSCs (Wilkinson et al. Nature 571:117;2019).Since the same few base hematopoietic culture media have been used for decades, we set out to develop a culture medium specifically for ex vivo expansion of human HSCs. In a Design of Experiments approach, media constituents were systematically varied and each iteration evaluated with the goal to maximize ex vivo expansion of hematopoietic stem-progenitor cell (HSPC) immunophenotypes.
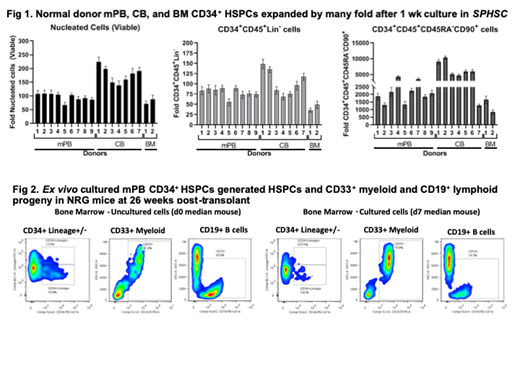
After 1 week (wk) culture of human mPB-, CB- or BM-derived CD34+ cells in optimized xeno-free, serum-free StemPro™ HSC Expansion Medium (Prototype) (SPHSC), supplemented with FLT3L, KITL, TPO, IL3, and IL6 (FKT36), there were on average 96-, 178- or 80-fold, respectively, greater numbers (yields) of viable nucleated cells, as compared to day (d)0 (Fig 1). Average yields of the CD34+CD45+Lin- HSPC immunophenotype were increased similarly, by 80-, 104- or 42-fold, respectively. Average yields of the CD34+CD45+CD45RA+CD90+ early HSPC immunophenotype were much more highly increased, by 2300-, 6047- or 1248-fold, respectively. CB-derived CD34+ cells typically generated greater fold increases in these parameters than did CD34+ cells from mPB or BM. In addition, we consistently observed (a) the presence of very early CD34+++CD90+ cells and (b) few Lin+ cells in the ex vivo cultured populations. Furthermore, compared to 2 different FKT36-containing commercial standard media, SPHSC supported statistically greater yields of nucleated cells, CD34+CD45+Lin- HSPCs and CD34+CD45+CD45RA+CD90+ early HSPCs.
We focused on the challenge of expanding HSCs from mPB, the most common clinical source. Yields of nucleated cells, CD34+CD45+Lin- HSPCs, and CD34+CD45+CD45RA+CD90+ early HSPCs expanded progressively from d4-d10. Transplant of 1 wk-cultured mPB CD34+ HSPCs generated donor cell dose-dependent increases in %hCD45+mCD45- cells in sublethally-irradiated NRG mouse bone marrows and spleens at 26 wks post-transplant. Human HSPCs and CD33+ myeloid and CD19+ lymphoid progeny were present in hematopoietic organs of the transplanted mice (Fig 2). The long-term (LT)-HSC engraftment capacity of the entire 1 wk-cultured cell population was 10-fold greater than at d0. These results for 1 wk ex vivo expansion of mPB CD34+ cells in SPHSC are similar or superior to reported 12d or 15d expansions of CB CD34+ cells in cytokine-supplemented standard media containing UM171 or SR1 (Fares et al. Science 345:1509;2014; Cohen et al. Biol Blood Marrow Transplant 24:S190;2018; Wagner et al.Cell Stem Cell 18:144;2016). Thus, SPHSC medium may by itself be impactful in clinical transplantation and gene therapy and would also be an optimized platform medium for additional approaches to further enhance ex vivo expansion of human HSCs for transplant and gene therapies.
Sei:Thermo Fisher Scientific Inc: Employment. Harris-Becker:Thermo Fisher Scientific Inc: Employment. Kaur:Thermo Fisher Scientific Inc: Employment. Vemuri:Thermo Fisher Scientific Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal