Introduction: Neurotoxicity is a major adverse event (AE) of CAR-T therapy with diverse presentation. When severe, it can be fatal, and may lead to neurologic sequelae as well as contribute to increased health care utilization, driving up cost of therapy. In clinical trials, the most common neurologic AEs with axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tis-cel) included encephalopathy, headache, tremor, dizziness, aphasia, delirium, insomnia and anxiety. Fatal and serious cases of cerebral edema, leukoencephalopathy and/ or seizures have been reported with both agents. In this real-world analysis, we reviewed post-marketing case reports from the FDA Adverse Events Reporting System (FAERS). Database involving axi-cel or tis-cel for large B cell lymphoma (LBCL), with the dual objectives of characterizing the various components of neurotoxicity and assessing the association of neurological (neuro) AEs with demographic and treatment factors as well as with other AEs reported with CAR-T cell therapy use.
Methods: The FAERS database contains anonymized reports of product-related AEs, classified using the Medical Dictionary for Regulatory Activities (MedDRA) and categorized as serious or non-serious. The FAERS database was queried for cases involving axi-cel or tis-cel (and their respective trade names) from the FDA approval date for the LBCL indication (October 18, 2017 for axi-cel; May 1, 2018 for tis-cel) through March 31, 2019. Cases were excluded if patient age was unknown or if the case was reported outside the US. Of all patients reported to have neuro AEs, the frequency of various components was collected. The association of neuro AEs with patient age, concomitant AEs and key lab abnormalities were evaluated by Fisher's exact test, using a two-sided α=0.05 to determine statistical significance. Median age in each subgroup was compared using the Mann-Whitney U test.
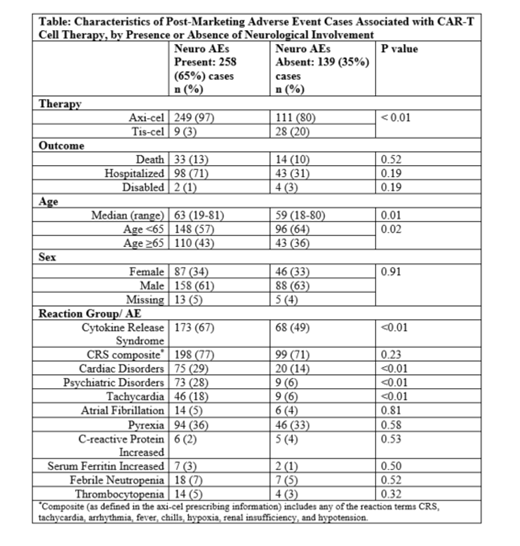
Results: In the 397 case reports identified, the majority of reactions (376, 95%) were classified as serious. Overall, 258 (65%) were reported to have neuro AEs, with "neurotoxicity" reported in 170 cases (66%); encephalopathy including CAR-T cell-related, metabolic and toxic in 92 cases (36%); seizures including status epilepticus, myoclonus and partial seizures in 12 cases (5%); stroke including cerebrovascular accident, hemiparesis, basal ganglia, brain stem, cerebellar and cerebral infarcts, motor dysfunction, facial and cranial nerve paralysis in 13 cases (5%); speech disorders including terms of aphasia, dysarthria, speech and language impairment in 55 cases (21%); amnesia and memory impairment in 18 cases (7%); brain or spinal cord edema and increased intracranial pressure in 6 cases (2%). Peripheral neuropathy was reported in 5 cases (2%). Symptoms of headache, tremors, dizziness and somnolence were reported in 30 (12%), 41 (16%) 3 (1%) and 34 (13%) cases respectively. Confusional state, delirium or agitation were reported in 61 cases (24%).
Neuro AEs were associated with use of axi-cel vs. tis-cel (69% vs. 24%, p <0.01) and with age ≥65 years (43% vs. 36% in age <65, p=0.02) but did not impact death or hospitalization (Table). Neuro AEs were associated with cytokine release syndrome (CRS, 67% vs. 49% without, p<0.01) as well as with cardiac AEs including tachycardia (p<0.01). There was an association of psychiatric AEs with neuro AEs though some (delirium, agitation, hallucination) may be considered a part of the neuro AEs. There were no associations of febrile neutropenia, thrombocytopenia, serum ferritin and C-reactive protein with neuro AEs (all p values>0.05).
Conclusions: Neuro AEs were common with CAR-T cell therapy in the real world and largely resembled those reported in clinical trials. Neuro AEs were associated with the agent used, age ≥65 as well as the presence of CRS, cardiac and psychiatric AEs but not with any of the laboratory values studied. The limitations of this study include its retrospective nature, potential under-reporting to FAERS and the relatively small number of patients in the tis-cel group due to its later approval and shorter time available for uptake compared to axi-cel. Despite these limitations, our findings can serve to inform clinicians' decision making when treating adult patients with CAR-T cell therapy. Further research is needed to better discern the etiopathology and biomarkers of neuro AEs in CAR-T cell therapy.
Gajra:Cardinal Health: Employment. Zettler:Cardinal Health: Employment. Phillips Jr.:Cardinal Health: Employment. Klink:Cardinal Health: Employment. Kish:Cardinal Health: Employment. Mehta:Cardinal Health: Employment. Feinberg:Cardinal Health: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal