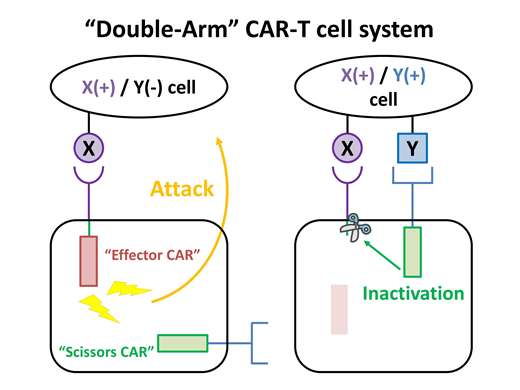
【Introduction】 CAR-T cell therapy is an attractive methodology in the field of cancer immunotherapy. Anti-CD19 CAR is used for treating refractory B cell malignancies and shows excellent therapeutic effects. However, there are several disadvantages to be overcome in this therapy. "On-target / off-tumor" effect is one of such adverse effects. Current CAR-T cell therapy targets single cell-surface molecule, which leads to damage of normal cells expressing the target protein. Improvement of target-cell-specificity is one of important issues in CAR-T cell therapy to avoid this serious adverse effect. Here, we show the protease-mediated "Double-Arm" CAR-T cell system (Figure), which improved the specificity of CAR-T cell therapy by recognizing two distinct cell-surface proteins. We designed two types of CAR: "Effector CAR" and "Scissors CAR" (Figure, left panel). The "effector CAR" is constituted of a single chain Fv fragment (scFv) targeting a cell-surface protein (protein X) on tumor cells, Human Immunodeficiency Virus protease (HIVPR) recognition polypeptide sequence, and a functional domain of CD3-zeta. The "scissors CAR" is constituted of a recognition portion targeting another protein (protein Y) and HIVPR. The HIVPR induces cleavage of the recognition polypeptide sequence in the effector CAR leading to inactivation of the effector CAR when the CAR-T cells contact with cells expressing both protein X and Y (Figure, right panel).
【Material and Methods】 For proof of principle, we first constructed "anti-CD19 mCherry CAR" harboring mCherry fluorescence protein in the cytoplasmic region under the HIVPR recognition polypeptide sequence. Also, we constructed "anti-CD19 scissors CAR" and "anti-HER2 scissors CAR". To analyze the target-cell-dependent cleavage of mCherry CAR, 293T cells expressing these CARs were co-cultured with target cells, including K562 (CD19-, HER2-), Raji (CD19+, HER2+), or SK-BR-3 (CD19-, HER2+). To obtain target cells expressing both CD19 and HER2, Raji and SK-BR-3 cells were molecularly manipulated. (1) To evaluate efficiency of this system, after co-cultivation of CAR-transduced 293T cell and target cells (K562, Raji, SK-BR-3), the localization of mCherry was examined under the microscopy and Western blotting. (2) To assess the T cell activation, we constructed "anti-CD19 effector CAR" and established Jurkat cells expressing both the "effector CAR" and the "anti-HER2 scissors CAR". These cells were co-cultured with wild type or engineered Raji or SK-BR-3 cells. T cell activation was analyzed with flowcytometric analysis and IL-2 mRNA expression measured with qRT-PCR.
【Results】 (1) Transduced "anti-CD19 mCherry CAR" was detected as a membrane-bound protein in 293T cell. Co-cultivation of 293T cell expressing both the "anti-CD19 mCherry CAR" and "anti-HER2 scissors CAR" with engineered Raji cells expressing both CD19 and HER2 induced cleavage of the recognition site and translocation of the mCherry from the membrane to cytoplasm. In addition, the cleavage was inhibited by a HIVPR inhibitor, Saquinavir. These results suggested that this novel system would regulate CAR-T cell activities through HIVPR-mediated cleavage of the "effector CAR" in vitro. (2) Jurkat cells expressing "anti-CD19 effector CAR" were activated through the target-cell-dependent manner. In addition, "anti-HER2 scissors CAR" attenuated T cell activation driven by "anti-CD19 effector CAR" when Jurkat cells expressing both the "anti-CD19 effector CAR" and the "anti-HER2 scissors CAR" contacted with the target cells expressing both CD19 and HER2.
【Discussion】 This is a novel protease-mediated controllable CAR system. The "scissors CAR" regulated activity of CAR-T cells depending on expression pattern of target molecules on the target cells. Our "Double-Arm" CAR-T cell system (Figure) would improve target specificity. It would attenuate the adverse effects and contribute to expansion in application of CAR-T cell therapy other than B cell malignancies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal