Background:
Adoptive transfer of CD4+CD25+FOXP3+ regulatory T cells (Tregs) effectively prevents conventional T cell (Tcons) mediated Graft versus Host Disease (GvHD) while it does not impair graft-versus-leukemia effect in haploidentical hematopoietic cell transplantation (haplo-HCT). Moreover Treg immunotherapy promotes fast donor T cell recovery after transplant. Recent studies showed that mouse bone marrow (BM) Tregs localize in the hematopoietic stem cell (HSC) niche, where they contribute to HSCs maintenance and promote donor engraftment and B cell lymphopoiesis. We are investigating if human Tregs promote B cell reconstitution and immunity in preclinical models and in haplo-HCT patients.
Methods:
Human sample analysis: B cell reconstitution was analysed monthly by FACS in BM and peripheral blood (PB) samples from 66 patients who underwent either Treg/Tcon haplo-HCT (45 patients), or T-cell depleted haplo-HCT (8 patients) or haplo-HCT with post-transplant cyclophosphamide (PTCy, 13 patients). Diagnosis was acute leukemia in 52 patients, lymphoma in 11 and multiple myeloma in 3. PB total immunoglobulin (Ig), anti-Cytomegalovirus (CMV) IgM and CMV viremia were monitored.
Humanized mouse model: donor derived human Tregs and purified CD34+ HSCs were co-infused in sublethally irradiated (2 Gy) immune-deficient NSG mice. Donor engraftment and B cell reconstitution were analysed by FACS and histology.
Results:
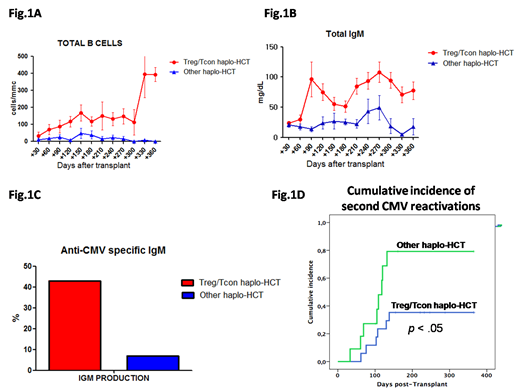
B cell reconstitution was faster after Treg/Tcon haplo-HCT when compared to other haplo-HCT protocols. B cell counts were higher in PB of patients that received Treg/Tcon haplo-HCT (p<.05) and were comparable to those of healthy subjects by 4 months after transplant (117±148 cells/mm, Fig.1A). We could detect early frequencies of CD34+CD38+CD10+CD127+ common lymphoid progenitors, CD45+CD10+CD38+CD19- Pre/Pro-B, CD45+CD10+CD38+CD19+ Pre-B, and Pro-B cells in the BM of these patients, that resulted in an increased production of CD38+CD19+CD5-IgM+ immature B cells, CD38+CD19+CD5+IgM+ transitional B cells and CD19+CD20+ mature B cells. We used a mouse model of xenotransplantation to understand whether donor B cell reconstitution in Treg/Tcon haplo-HCT is boosted by a Treg-mediated effect on donor human HSCs. We found that infusion of human Tregs facilitated donor HSC engraftment. BM and PB human chimerism was increased (p<.05) in mice that received Treg infusion. BM histology revealed the presence of in vivo expanded human CXCR4+ Tregs in the femurs of Treg receiving mice 1 month after transplant. In the same samples we observed a lower number of CD34+ HSCs, suggesting BM Tregs facilitate donor HSC differentiation. Moreover, Treg treatment allowed human HSCs to localize preferentially in the epiphyseal areas of the femurs, where donor cells started to engraft (p<.04). When looking at donor B cell reconstitution, we found HSC-derived mature B cells rapidly abundant and easily detectable starting 30 days after HSC infusion in PB of Treg-treated animals (p<.05).
To further assess whether HSC-derived B cells were functional in patients that received Treg/Tcon haplo-HCT, we analysed total Ig production and specific responses to CMV reactivation. Post-transplant hypogammaglobulinemia was rapidly corrected in Treg/Tcon haplo-HCT patients. Total IgM were higher compared to other haplo-HCT protocols and reached normal levels by 3 months after transplant (96±155 mg/dL, Fig.1B). CMV reactivation rate was lower (47% vs 79%) and it occurred later after Treg/Tcon immunotherapy (51±19 vs 40±48 days). New production of anti-CMV specific IgM was documented in 43% of CMV seropositive patients 98±48 days after Treg/Tcon haplo-HCT, while anti-CMV specific IgM were almost undetectable after other haplo-HCT protocols within the first 6 months after transplant (Fig.1C). Such potent B cell responses coupled with T cell reconstitution resulted in a reduction of CMV second reactivations (Fig.1D p<.05) with no patient that died with CMV disease.
Conclusions:
Adoptive transfer of human Tregs boosts donor HSC engraftment and facilitates the reconstitution of functional HSC-derived donor B cells. Such results suggest that Treg/Tcon haplo-HCT patients could be early vaccinated after transplant. Treg/Tcon immunotherapy promotes control of infections and B cell immunity in patients undergoing haplo-HCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal