Background: Ibrutinib (ibr) is a small-molecular inhibitor of Bruton's Tyrosine Kinase (BTK), active in the treatment of various B-cell malignancies. B-cell receptor signaling blockade by BTK inhibition using ibr down regulates IRF4 (survival transcription factor) which is down regulated by lenalidomide (R) as well, suggesting possible synergistic effect on cell death. Higher doses of ibr (560-840 mg daily) have been used in combination regimens for MM with no significant dose-limiting toxicities (DLTs) but the combination of ibr and len has not been previously tested. We present safety and preliminary efficacy of the combination ibr, R, and dexamethasone (d) in RRMM patients.
Methods: This is a phase I dose escalation study (NCT03015792) of 28-day cycles of ibr (420 mg daily, 560 mg daily, 700 mg daily or 840 mg daily) days 1-28 in combination with R 25 mg PO days 1-21, and d 40 mg PO weekly utilizing a 3+3 dose-escalation design. Eligible RRMM patients had progression after ≥2 prior lines of treatment, measurable disease as per International Myeloma Working Group criteria, ECOG performance status ≤2, adequate bone marrow (BM) (absolute neutrophil count ≥1.0 x 109, platelets ≥50,000 cells/mm3 for patients with BM plasmacytosis <50% or ≥30,000 cells/mm3 for patients with BM plasmacytosis ≥50%), kidney (creatinine clearance ≥30 mL/min), and liver function [total bilirubin ≤1.5 x upper limit of normal (ULN), aspartate aminotransferase and alanine aminotransferase ≤3 x ULN], and PT/INR ≤1.5 X ULN. Treatment was to be continued till progressive disease (PD) or any unacceptable toxicity. The primary objective was to determine the maximum tolerated dose (MTD) of ibr+Rd in RRMM and secondary objective was to evaluate safety profile of this regimen.
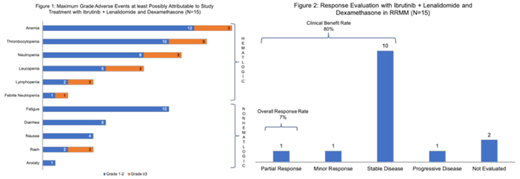
Results: As of July 26 2019, 18 patients had been enrolled in the trial. Three patients had to be replaced (2 at 700 mg cohort, 1 each due to withdrawal and ineligibility, and 1 at 840 mg due to withdrawal). Median age of all patients was 67 years (range 49-79) with 9 of the 15 evaluable patients being females. Evaluable patients as per ibr dose level included 3 at 420 mg, 3 at 560 mg, 3 at 700 mg, and 6 at 840 mg. Four out of 15 patients had high-risk cytogenetics. Median prior lines of treatment were 4 (range 2-13) and prior treatments included bortezomib in 87% (n=13), carfilzomib in 47% (n=7), ixazomib in 47% (n=7), lenalidomide in 87% (n=13), pomalidomide in 40% (n=6), thalidomide in 40% (n=6), daratumumab in 60% (n=9), and stem cell transplant in 53% (n=8). High risk cytogenetics [del 17p, t(4;14), t(14;16), t(14;20)] were noted in 4 of the 15 evaluable patients (27%). Median follow up for alive patients was 8.6 months (range 1.1-25.1 months) and the median number of treatment cycles was 2 (range 1-5). Most common reason for treatment discontinuation was PD (40%) followed by adverse events (AEs) (26.7%). Only 1 DLT possibly related to ibr was a grade 3 rash at the 840 mg dose. Grade 3/4 AEs at least possibly related to study treatment included anemia (n=3), thrombocytopenia (n=3), neutropenia (n=3), leucopenia (n=3), lymphopenia (n=2), febrile neutropenia (n=1), and rash (n=2). Overall, the most common all grade AEs included anemia (n=12), thrombocytopenia (n=10), fatigue (n=10), neutropenia (n=8), leucopenia (n=5), and diarrhea (n=5). (Figure 1) No treatment-related deaths were noted. Overall response rate (ORR) was 7% with partial response (PR) noted in 1 patient. Additionally, 1 patient achieved a minor response (MR) and 10 patients had stable disease (SD) for a clinical benefit rate (CBR) of 80%. (Figure 2) PD was noted in 1 patient and 2 patients did not get response assessment. Ibr 840 mg (daily) with R 25 mg (days 1-21) and d 40 mg weekly was considered the MTD of this regimen. The median progression-free survival (PFS) for the 15 evaluable patients was 4.5 months (95% CI: 1.8-not reached).
Conclusions: We report the first phase 1 trial of combining a BTK inhibitor with Rd in RRMM patients. MTD of ibr was determined as 840 mg (daily) in combination with R 25 mg (days 1-21) and d 40 mg weekly. This dose of ibr is consistent with some other trials showing the benefit of a higher dose of ibr in various regimens for treatment of B-cell malignancies. We noted this regimen to be well-tolerated without much high-grade AEs. Disease stabilization was noted in majority of patients. These data lay the basis for a larger trial in a more uniform cohort of patients to better define the efficacy of this regimen.
Ailawadhi:Celgene: Consultancy; Amgen: Consultancy, Research Funding; Pharmacyclics: Research Funding; Cellectar: Research Funding; Janssen: Consultancy, Research Funding; Takeda: Consultancy. Chanan-Khan:AbbVie: Research Funding; Xencor: Research Funding; Pharmacyclics: Research Funding; Merck: Research Funding; Jansen: Research Funding; Mayo Clinic: Employment; Ascentage: Research Funding; Millennium: Research Funding.
Ibrutinib is not FDA-approved for the treatment of multiple myeloma. The regimen of ibrutinib with lenalidomide and dexamethasone is not FDA-approved for the treatment of multiple myeloma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal