Background:
CyBorD (bortezomib, cyclophosphamide, and dexamethasone) is considered as an effective induction regimen in newly diagnosed light chain amyloidosis (AL) patients. Although a full dose of dexamethasone (Dex) leads to a higher response rate, the dose is strictly limited, usually because of fluid retention. Additionally, supervised administration of bortezomib weekly, if applied, increases the cost and inconvenience of treatment for service providers and patients.Ixazomib(IXA), an oral proteasome inhibitor, was reported highly effective as a single agent in relapsed or refractory AL amyloidosis. The objective of this observation is to evaluate the feasibility and efficiency of adding a lower dose of Dex to IXA (Id) as an upfront regimen in AL pts.
Patients and Methods:
Between 9/29/2018-4/1/2019, twenty-five newly diagnosed (ND) AL pts were sequentially enrolled. All AL pts had positive Congo Red staining in biopsy specimens confirmed by immunoelectron microscopy(IEM). Ixazomib 4mg D1,8,15 and Dexamethasone 10mg D1,8,15,22 were given for a 28-day schedule until disease progression or intolerance. Efficiency and safety profiles were assessed at the beginning of each cycle. Patients who had not achieved PR after 3 cycles received additional oral cyclophosphamide (50mg daily). The patients not achieving PR after 3 more cycles would switch to salvage therapies such as lenalidomide-base regimens or melphalan-based regimens. Upfront Id regimen can be prolonged for 2 cycles after a best hematological response (CR) has been achieved. The primary objective was to determine the response rate of this regimen and to evaluate the safety and tolerability of Upfront Id. Secondary objectives included PFS and OS.
Results:
Patients(n=25) received a median of 4 cycles (1-8) of Id regimen. Most patients were at late stage (20 pts in MAYO stage III, 4 pts in stage I and 1 pts in stage II). The interphase FISH analysis in BM plasma cells finds translocation t(11,14) in 8 pts.(Table 1)
All patients were evaluable for toxicity and twenty-four for the response. The ORR was 66.7%(16/24) post cycle 1 and 70.8%(17/24) post cycle 4. Best hematological response achieved to date of this study is CR in 10pts, VGPR in 5 pts. 5 of the 7 pts did not reach their hematological remission have a t(11,14) translocation.
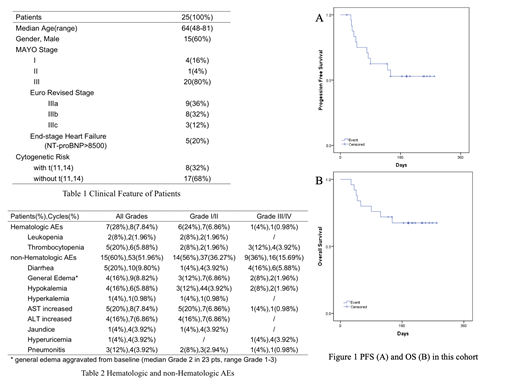
With a median of 197 days (34-281) follow-up, 68%(17/25) of the patients were still alive, and 41.7%(10/24) with their best hematological response. 4 of the patients died of sudden and the rest due to the progression of heart failure. (Figure 1)
According to CTC AE 5.0, Grade III/IV AEs (no. of pts) included: diarrhea 16%(4), thrombocytopenia 12%(3), general edema 8%(2), hypokalemia 8%(2), AST increased 4%(1), hyperuricemia 4%(1), and pneumonitis 4%(1). All the 4 patients with serious diarrhea quit therapy because of intolerance. (Table 2)
Conclusions:
Adding low dose dexamethasone to Ixazomib can induce rapid and profound hematological remission in newly diagnosed AL pts, especially in patients without t(11,14). This entirely oral, chemotherapy-free combination regimen ensures patients' compliance. The relatively low incidence of Grade III/IV AEs also makes the regimen seemingly a broad usage in MAYO stage III pts. However, diarrhea and thrombocytopenia in these patients still need awareness.
Ixazomib (Ixa) is the first oral proteasome inhibitor that approved for the use in patients with relapsed/refractory multiple myeloma (RRMM) in > 60 countries including US and China. In this single-center real-world study, we present the efficacy and safety profile of Ixazomib-based therapy as frontline therapy in patients with AL amyloidosis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal