
Background: The International Society of Amyloidosis validated the criteria of hematologic response to therapy in AL amyloidosis, based on the reduction of free light chain (FLC) concentration and serum and urine immunofixation. Complete response (CR) predicted the longest survival and was defined by negative serum and urine immunofixation (s&u-IFE) with a normal FLC ratio (FLCR). Subsequently, we and others showed that, in patients with a difference between involved and uninvolved FLC (dFLC) between 20 and 49 mg/L, achieving a dFLC <10 mg/L was associated with an improvement of overall and renal survival. This criterion was then proposed as a goal of therapy in the general population of patients with AL amyloidosis. These studies questioned whether the definition of CR should be reconsidered. In the present study, we compared the outcome of patients who obtained a profound reduction of FLC but do not qualify for CR with the outcome of subjects who reached CR.
Methods: The prospectively maintained database of the Pavia Amyloidosis Center was searched for patients who were newly-diagnosed between 2004 and 2018 and who reached at least one the following endpoints 6 months after treatment initiation: 1) complete response (CR) or, in patients who do not qualify for CR, 2) normalization of involved-FLC concentration (normal-iFLC group), dFLC <10 mg/L (dFLC10 group) and normalization of FLCR (normal-FLCR group). We calculated overall survival (OS) and time to next line of therapy or death (TNTD) from the time of response assessment (6 months after treatment initiation).
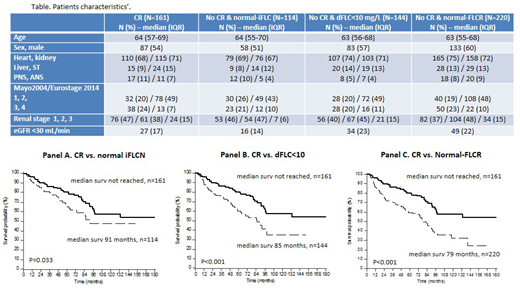
Results: A total of 434 patients (Table) were included, that were divided into the following groups: CR (161), normal-iFLCN (114), dFLC10 (144) and normal-FLCR (220). As expected, there was a partial overlap between the non-CR groups. No difference was seen between the CR and all the other groups in baseline variables: age, sex, organ involvement, Mayo 2004/European staging, and renal staging. Treatment was bortezomib-based in 261 subjects, melphalan/dexamethasone (MDex) in 128, IMiDs-based in 21, autologous stem cell transplant in 14, and therapy for IgM clones in the remaining 10 subjects.
The reasons for not qualifying for CR in the normal-iFLC group was abnormal FLCR only in 4 (3%), positive s&u-IFE only in 81 (72%), and both in 29 (25%). The reason for not qualifying for CR in the dFLC10 group was abnormal FLCR only in 4 (3%), positive s&u-IFE only in 104 (72%) and both in 36 (25%).
The median follow-up of living patients was 60 months. Patients who achieved CR enjoyed a significantly longer OS (58% alive at 10 years) compared to patients who reached any of the other endpoints but did not qualify for CR (Figure). Also median TNTD was significantly longer in patients who obtained CR (75 months) compared to the normal-iFLC group (33 months, P<0.001), the dFLC10 group (39 months, P<0.001), and the normal-FLCR group (24 months, P<0.001).
We then evaluated whether achieving a normal iFLC or a dFLC <10 mg/L was associated with a better survival among patients who did qualify for CR. In the CR group, 103 (64%) patients achieved a normal iFLC and 86 (53%) a dFLC <10 mg/L. There was no significant difference in OS and TNTD between subgroups of patients in CR according to iFLC or dFLC response.
Conclusion: Undetectable clonal disease by both negative serum and urine IFE and normal FLCR (CR) is associated with best survival in AL amyloidosis and should be the goal of therapy if tolerability and patient frailty allow. However, it is possible that novel immunotherapies, such as daratumumab, affect the concentration of uninvolved FLC, thus interfering with dFLC and FLCR, and this study should be validated in patients exposed to these agents. Approximately 40% of patients in CR relapse and eventually die. New sensitive tools to identify residual clonal disease (mass-spectrometry on serum and urine, next generation sequencing and next generation flow cytometry on bone marrow) are urgently needed to detect these patients and intervene before relapse.
Milani:Janssen: Honoraria; Pfizef: Honoraria. Palladini:Janssen-Cilag: Other: Travel grant; Janssen-Cilag: Honoraria; Celgene: Other: Travel grant; Sebia: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal