Background
PI-based therapy is a standard of care for non-transplant NDMM pts. However, long-term treatment, which is associated with improved outcomes, is often challenging in the RW. This may be due to a number of factors, including the burden of repeated intravenous (IV)/subcutaneous (SC) administration, distance from treatment center, comorbidities, and toxicity (e.g. peripheral neuropathy [PN] with btz). With the aim of increasing PI-based treatment adherence and duration while maintaining quality of life (QoL), the US MM-6 RW, community-based study (NCT03173092) investigates a transition from IV/SC btz-based induction to all-oral ixazomib-based therapy (ixazomib-lenalidomide-dexamethasone, IRd). We report efficacy and safety, plus adherence and electronic pt-reported outcomes (ePRO) compliance data, for the first 55 pts.
Methods
Non-transplant NDMM pts (transplant-ineligible or transplant delayed >24 mos) with ≥stable disease (SD) after 3 cycles of a btz-based induction are being enrolled at 23 community sites to receive IRd (ixazomib 4 mg, d 1, 8, 15; lenalidomide 25 mg, d 1-21; dexamethasone 40 mg [20 mg in pts aged >75 yrs], d 1, 8, 15, 22) for up to 26 x 28-d cycles or until progression/toxicity. Pts complete ePROs every cycle to assess QoL/treatment satisfaction, and a monthly medication adherence survey via a wearable device/smartphone. The primary endpoint is progression-free survival (PFS); key secondary endpoints include partial (PR), very good partial (VGPR), and complete (CR) response rates, and duration of therapy.
Results
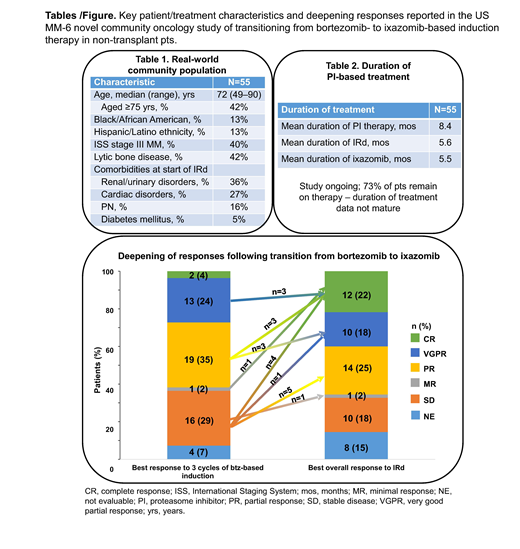
As of April 1 2019, 55 pts had been enrolled at 16 sites. Median age was 72 (range 49-90) yrs, with 76% classified as elderly (≥65 yrs); 47% were male. Key characteristics of this RW population are summarized in Table 1. Comorbidities/concurrent medical conditions at the start of IRd therapy were extensive and included hypertension (51%), anemia (44%), fatigue (42%), renal and urinary disorders (36%), gastroesophageal reflux disease (31%), cardiac disorders (27%), constipation (27%), nausea (24%), and PN (16%); 91% of pts were receiving concomitant medications. At data cutoff, with 40 (73%) pts remaining on therapy, median duration of PI therapy, including prior btz-based induction, was 6.9 mos (mean 8.4 mos) (Table 2). Median duration of IRd treatment was 4.0 mos (median 5 cycles; mean 5.6 mos, 6.6 cycles), with pts having received up to 17.3 mos (18 cycles) of therapy to date.
After 3 cycles of btz-based induction, the ≥VGPR rate was 27%, with 4% ≥CR; overall response rate (ORR) was 62%. With IRd therapy, the ≥VGPR rate was 40%, with 22% ≥CR; ORR was 65% (15% not evaluable). Following transition from btz-based induction to IRd, 21 pts (36%) had deepened responses (18% increase in ≥CR rate), including 3 VGPR to CR, 3 PR to CR, 1 MR to CR, 4 SD to CR, 3 PR to VGPR, 1 SD to VGPR, 5 SD to PR, and 1 SD to MR (Figure). With limited follow-up, and enrollment ongoing, 3 pts had progressed and one had died at data cutoff. The preliminary 6-mo PFS rate (95% CI) was 91% (74-97%) from start of IRd and 96% (84-99%) from start of btz-based induction. Average compliance with completing issued ePRO questionnaires during IRd treatment was 96% (61 pts; data cutoff July 8, 2019). Patients recorded their monthly medication adherence for the previous 4 weeks; 81% of evaluable pts (n=32) in cycle 1, 81% in cycle 2 (n=27), 77% in cycle 3 (n=22), 96% in cycle 4 (n=24), and 94% in cycle 5 (n=18) (n<11 [20% of pts] beyond cycle 5) reported 'excellent'/'very good' adherence. During IRd treatment to date, 87% of pts had adverse events (AEs); 44% had grade 3/4 AEs, including grade 3 pneumonia (7%), syncope (7%), and diarrhea (5%) occurring in >2 pts. AEs led to study drug modification in 47% and discontinuation in 4% of pts; 29% had serious AEs. PN occurred in 25% (4% grade 3) and led to dose modification in 13% of pts. There were no on-study deaths (i.e. occurring <30 d of last dose).
Conclusions
These preliminary data in mostly elderly, comorbid, NDMM pts treated in the RW, community setting indicate the feasibility, tolerability, and efficacy of transitioning to IRd after 3 cycles of btz-based induction. Toxicities appeared similar to previous ixazomib studies. With 73% of pts remaining on therapy and enrollment continuing, duration of therapy is promising, substantial improvements in response have been seen, and ePRO compliance/ medication adherence is high, indicating the feasibility and value of these evaluations.
Rifkin:Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Noga:Takeda: Employment. Yimer:Amgen: Consultancy; Puma Biotechnology: Equity Ownership; Clovis Oncology: Equity Ownership; Celgene: Honoraria; Seattle Genetics: Honoraria; Janssen: Speakers Bureau; AstraZeneca: Speakers Bureau. Girnius:Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Birhiray:Puma: Consultancy, Research Funding, Speakers Bureau; Alexion: Consultancy; Kite Pharma: Honoraria; Bayer: Honoraria; Helsin: Honoraria; Incyte: Research Funding, Speakers Bureau; Seattle Genetics: Honoraria; Pfizer: Speakers Bureau; Abbvie: Consultancy, Honoraria; Celgene: Honoraria; AstraZeneca: Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Sanofi Oncology: Speakers Bureau; Lilly: Speakers Bureau; Genomic Health: Speakers Bureau; Amgen: Honoraria, Speakers Bureau; BMS: Speakers Bureau; Tessaro: Speakers Bureau; Exelexis: Speakers Bureau; Clovis Oncology: Speakers Bureau; Jansen Bioncology: Consultancy, Speakers Bureau; Coheris: Honoraria; Takeda: Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Speakers Bureau. Yasenchak:Seattle Genetics: Consultancy; BMS: Consultancy. Lyons:Texas Oncology: Equity Ownership; Amgen: Consultancy; McKesson: Other: Leadership. Whidden:Takeda: Employment. Schlossman:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Wang:Millennium Pharmaceuticals, Inc., Cambridge, MA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Boccia:DSI: Speakers Bureau; AstraZeneca: Speakers Bureau; Amgen: Speakers Bureau; Celgene: Speakers Bureau; Genentech: Speakers Bureau; AMAG: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal