Background:
Achievement of MRD-negative status in MM patients has been demonstrated to be associated with better outcome. EuroFlow MM-MRD has been a global standard for MRD detection by MFC. In Japan, EuroFlow is limited to research use only, because the dynamics of the population have raised concerns about increasing health care expenditure in Japan. Therefore, it is desirable to develop an inexpensive FCM method with equivalent sensitivity to EuroFlow for detecting MRD.
Aims:
In this study, we analyzed the number and percentage of total plasma cells (PC), abnormal PC with EuroFlow MM-MRD and 10-color-MFC in 50 MM patients.
Patients and Methods:
Bone marrow samples collected from 50 MM patients were subjected to MRD detection by EuroFlow MM-MRD and 10-color-MFC simultaneously. EuroFlow MM-MRD was performed according to EuroFlow standard operating procedure. 10-color MFC was performed at BML corporation. Briefly, CD38 multiepitope FITC, CD138 V450, CD45 V500-C, CD56 PE, CD19 APC-H7, CD27 APC R700, CD81 BV605, CD117 PE-Cy7, cIgk APC and cIgl PerCP-Cy5.5 of monoclonal antibody were used according to the ICCS(International Clinical Cytometry Society) guidelines. Data were analyzed with FACSuite ver 1.2.1(Becton Dickinson) using a FACSLylic(Becton Dickinson). Wilcoxon signed-rank test was used for the comparison of paired variables.
Results:
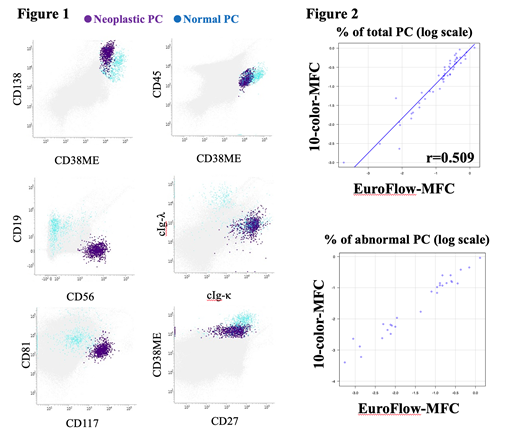
The representative example of 10-color-MFC were shown in figure 1. We first compared the percentage of normal plasma cells in bone marrow. The percentage of normal plasma cells analyzed with 10-color-MFC and EuroFlow were 0.287% (range 0.001-1.0322%) and 0.257%(range0.0002-1.37%), respectively. The good correlation between them was observed (r=0.509). The percentage of myeloma cells determined with 10-color-MFC and EuroFlow were 0.0705%(range 0-0.9086%) and 0.0940% (range 0-1.3000%), respectively. Quantification of abnormal plasma cells were highly similar between 2 tests (r=0.977, figure 2). To compare the percentage of abnormal plasma cells analyzed with 10-color-MFC and EuroFlow, p-value determined with Wilcoxon signed-rank test was 0.0069 in 50 sample. However, when total bone marrow cells were 3 million cells, limit of detection (LOD) and limit of quantification (LOQ) in 10-color MFC were defined as 0.001% and 0.0017%, respectively. The correlation in samples with less than 3 million of bone marrow cells was not good (p=0.424). Only when 3 million or higher number of bone marrow cells were analyzed, better correlation is thought to be observed (p=0.0001). High concordant analytical results were observed with an overall qualitative concordance of 95%.
Conclusion:
Our 10-color-MFC demonstrated high sensitivity to detect MRD with good correlation and also inexpensive (about 100 dollars as of July 2019). However, 3 million or higher number of bone marrow cells is inevitable to demonstrate good correlation. In the country where high medical expenditure is not acceptable, our inexpensive MFC is considered as good alternative methods to detect MRD in MM patients.
Tsukada:Takeda Pharmaceutical Co., Ltd.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Celgene: Honoraria; Ono Pharmaceutical: Honoraria; Sanofi: Honoraria; Kyowa Kirin: Honoraria; Chugai Pharmaceutical Co.,Ltd: Honoraria; Fujimoto Pharmaceutical: Honoraria; Ohtsuka Pharmaceutical: Honoraria; Asahi Kasei Pharma Corporation: Honoraria; MOCHIDA PHARMACEUTICAL CO., LTD.: Honoraria. Suzuki:Ono: Research Funding; BMS: Honoraria, Research Funding; Takeda: Honoraria; Janssen: Honoraria; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal