Introduction: The role of serum free light chain (sFLC) assay for monitoring immunoglobulin (Ig) secretory multiple myeloma (Ig-MM) remains an area of debate. IMWG guidelines (Durie et al. Leukemia 2006) recommend the use of electrophoresis (EP) and immunofixation (IF) for definitions of response and progressive disease (PD), while the measurement of sFLC is required only to fulfill criteria for stringent complete response.
Aims: We aimed at evaluating the prognostic impact of sFLC levels during monitoring of Ig-MM.
Methods: We analyzed all consecutive Ig-MM patients (pts) who received at our Centre a first line novel agent-based therapy between October 2006 and December 2018, and for whom sFLC data by BN II nephelometer, along with serum and urine EP plus IF, were available at diagnosis and every 3-4 months until the last contact date. Criteria for response and PD were those established by the IMWG. IMWG criteria for PD in oligo/non-secretory MM were used to identify an increase in sFLC levels. sFLC escape (sFLCe) was defined as an increase in sFLC, without any IMWG criteria of PD. To evaluate the prognostic role of rising sFLC, sFLCe was considered as an event for outcomes after relapse. Second time to progression/sFLCe (2nd TTPe) was the time from the date of first PD or sFLCe to that of second PD or sFLCe, while OS after progression/sFLCe (OS after Pe) was calculated from first PD or sFLCe to the date of death.
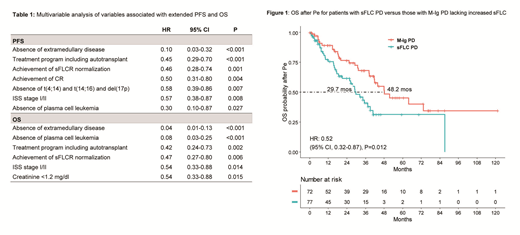
Results: A total of 339 pts were identified. At baseline, 311 (92%) pts had an abnormal sFLC ratio (sFLCR) and 231 (68%) a sFLC measurable disease defined by an abnormal sFLCR and involved sFLC ≥100 mg/L. First-line treatments included proteasome-inhibitors (PI) (42%), immunomodulators (IMiDs) (13%), or both these agents (45%). Autotransplant was performed in 48% of pts. Overall, 148 (44%) pts achieved a complete response (CR) and 198 (60%) achieved (or maintained) a normal sFLCR. With a median follow-up of 54.1 (IQR 25.0-83.7) months (mos), median PFS and OS were 35.9 and 97.9 mos. The presence at baseline of a sFLC measurable disease was associated with worse PFS (median 31.5 vs 45.6 mos, HR=1.58, p=0.004) and OS (64.5% vs 83.3% at 54 mos, HR=1.98, p=0.003), while sFLCR normalization predicted for extended PFS (median 45.3 vs 16.8 mos, HR=0.21, p<0.001) and OS (80.4% vs 43.7% at 54 mos, HR=0.27, p<0.001) as compared with persistence of an abnormal sFLCR. Achieving a normal sFLCR maintained an independent prognostic value by multivariable analysis for both PFS (HR=0.46, p=0.001) and OS (HR=0.47, p=0.006) (table 1). Overall, 175 (52%) pts experienced PD defined by either increased monoclonal-Ig (M-Ig) (137 pts, 78%) or development of organ damage without changes in M-Ig (38 pts, 22%). A preceding or concomitant increase in sFLC was observed in 65 (47%) of the 137 pts with M-Ig PD and in 8 (21%) of the 38 pts without M-Ig changes. Among the whole study population, 31 (9%) pts experienced a sFLCe and in 26 (84%) of these pts a Ig-M PD occurred after a median time of 42 (IQR 31-133) days. Overall, 160 (47%) pts started a second-line therapy. To evaluate the role of increased sFLC in refining the definition of PD, pts who relapsed with rising sFLC as well as those with sFLCe (both referred to as sFLC PD), were compared to pts who relapsed with or without Ig-M changes, but lacking increased sFLC. In comparison with other patterns of relapse, a sFLC PD was associated with more advanced age (p=0.009) and higher rates of creatinine levels ≥1.2 mg/dl (p=0.048), del(17p) positivity (p=0.008), and sFLC evaluable disease (p<0.001) at baseline. Main features at relapse for this latter group were a lower Hb level (p=0.004) and a higher rate of creatinine ≥1.2 mg/dl (p=0.041). Median 2nd TTPe was 14.1 mos for pts with PD without any M-Ig and/or sFLC changes, 20.3 mos for those with sFLC PD, and 26.1 mos for those with only increased M-Ig (p=n.s.). Conversely, curves of OS after Pe were significantly different between these subgroups, with median values of 28.8, 29.7 and 48.2 mos (p=0.019), respectively. Notably, sFLC PD was confirmed to adversely affect OS after Pe in comparison with M-Ig PD without increased sFLC (HR=0.52, p=0.012) (figure 1), suggesting a more aggressive relapse.
Conclusions: Our results highlight the prognostic value of sFLC during monitoring of Ig-MM, and support the inclusion of the sFLC assay along with EP and IF for defining response and progression.
Tacchetti:Oncopeptides: Honoraria, Speakers Bureau; Celgene: Honoraria, Speakers Bureau; BMS: Honoraria; Amgen: Honoraria, Speakers Bureau; Janssen: Honoraria; Takeda: Honoraria, Speakers Bureau. Zamagni:Sanofi: Honoraria, Other: Advisory Board, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Amgen: Honoraria, Other: Advisory board, Speakers Bureau; BMS: Honoraria, Other: Advisory Board, Speakers Bureau; Celgene Corporation: Honoraria, Other: Advisory board, Speakers Bureau; Janssen: Honoraria, Other: Advisory board, Speakers Bureau. Pantani:Takeda: Honoraria; BMS: Honoraria; Janssen: Honoraria; Celgene: Honoraria; Amgen: Honoraria. Mancuso:Amgen: Honoraria; Janssen: Honoraria; Takeda: Honoraria; Celgene: Honoraria, Speakers Bureau. Zinzani:CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees; IMMUNE DESIGN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CELGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PORTOLA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANOFI: Consultancy; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; VERASTEM: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Cavo:bms: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; novartis: Honoraria; sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal