Introduction
Ibrutinib is an oral irreversible inhibitor of Bruton's tyrosine kinase for treatment of chronic lymphocytic leukemia (CLL). Ibrutinib has demonstrated superior efficacy for patients with TP53 aberration or relapsed/refractory (R/R) CLL; and more recently superior progression free survival (PFS) has been demonstrated compared to chemoimmunotherapy as first line therapy. However, knowledge about the outcomes and adverse events (AE) upon ibrutinib among patients at a population-based level are still limited. The aim of the here presented study is to explore outcomes of ibrutinib treatment in a population-based cohort of patients with CLL treated with ibrutinib in Denmark.
Methods
In this retrospective study, patients from 8 hospitals in Denmark, who were diagnosed with CLL and treated with ibrutinib from April 2014 until February 2019 were included. Medical records were retrospectively reviewed to obtain information. Patients receiving ibrutinib within clinical trials were excluded. Overall survival (OS) was defined as time from ibrutinib start to death from any cause while PFS was defined as time from ibrutinib start to progression or death from any cause. PFS and OS were analyzed with the Kaplan-Meier method while cumulative incidence was calculated with the Aalen-Johansen estimator.
Results
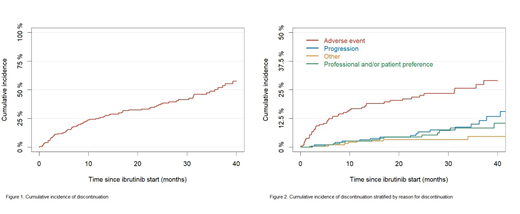
In total, 205 patients with CLL receiving ibrutinib treatment were identified from hospital records and registries. The median follow-up was 21.4 months (IQR, 11.9-32.8) and the median time on ibrutinib was 16.8 months (IQR, 6.0-28.1). The median age at treatment initiation was 72.8 years (IQR, 65.7-77.8), 128 (62.4%) were male, and 111 (63.4%) were Binet stage B/C at treatment initiation out of 175 with available information regarding clinical stage. Thirty-nine (19.0%) received ibrutinib as first-line, and 166 for R/R CLL with a median of 2 (range, 1-8) prior treatment regimens. Information on TP53 aberration was available for 149 and regarding IGHV mutation for 147 patients, 111 (74.5%) had TP53 aberration and 107 (72.8%) were IGHV unmutated. Eighty-six patients (42.0%) discontinued ibrutinib during follow-up with a median time until discontinuation of 9.3 months (IQR, 3.0-23.2). Forty-seven (54.7%) discontinued due to AEs, 19 (22.1%) due to progression (12 had progression of CLL and 7 had Richter's transformation) while the remaining 20 (23.2%) discontinued due to other reasons. The estimated cumulative incidence of discontinuation at 12 months was 24.8% (95% CI: 18.6-30.9). The estimated OS after 12 and 24 months was 88.8% (95%CI: 84.3-93.3) and 76.8% (95%CI: 70.4-83.2) and the estimated PFS after 12 and 24 months was 87.3% (95%CI: 82.5-92.1) and 72.4% (95%CI: 65.5-79.2).
One hundred and eighty-eight (91.7%) experienced at least one AE, among these 45 (23.9%) experienced a grade 3+. The most common AEs were hemorrhage (tendency to bruise, epistaxis etc.) which occurred in 86 (42.0%) of all and musculoskeletal and connective tissue disorders (arthralgia, myalgia etc.) which occurred in 82 (40.0%). Thirty-one (15.1%) patients experienced atrial fibrillation while on ibrutinib and 14 (6.8%) developed hypertension.
One hundred and thirty-seven patients (66.8%) had at least one infection and among these 80 (58.4%) were hospitalized with an infection. The most common infections were lower respiratory tract infections and urinary tract infections that occurred for 88 (42.9%) and 41 (20.0%). The estimated cumulative incidence for any infection was 58.9% (95%CI: 52.0-65.9) at 12 months.
Conclusion
This is the first study describing outcomes for a population-based cohort of CLL patients treated with ibrutinib in Denmark. Real-world studies are warranted, to confirm the results from clinical trials. In this study, patients appear to have comparable OS and types of AE compared with the RESONATE trial. Differences in frequency of AEs compared to the clinical trial may reflect the focus of clinicians in routine practice. Discontinuation in this cohort was higher compared to clinical trials but comparable to previously reported real-world studies. While ibrutinib can be safely managed in routine clinical practice, this study demonstrates that a quarter of patients discontinue treatment due to mainly AEs. Further patient training and information, and in some instances personalized treatment with other targeted agents based on adverse event profile, may improve treatment adherence.
Aarup:Research Committee, Rigshospitalet: Research Funding. Enggaard:Abbie: Other: Advisory board; Gilead: Other: Advisory board; Janssen: Other: Advisory board. Frederiksen:Gilead: Research Funding; Abbvie: Research Funding; Janssen Pharmaceuticals: Research Funding; Novartis: Research Funding; Alexion: Research Funding. Niemann:Novo Nordisk Foundation: Research Funding; AstraZeneca: Consultancy, Other: Travel Grant, Research Funding; Sunesis: Consultancy; Acerta: Consultancy; CSL Behring: Consultancy; Roche: Other: Travel Grant; Janssen: Consultancy, Other: Travel Grant, Research Funding; Gilead: Other: Travel Grant; Abbvie: Consultancy, Other: Travel Grant, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal