BACKGROUND
Novel agents have changed the treatment landscape in chronic lymphocytic leukemia (CLL), however little has been reported about real-world treatment sequence patterns and associated outcomes post-introduction of novel agents. Studies have demonstrated that venetoclax is effective for patients (pts) who have discontinued ibrutinib, however treatments and outcomes post-venetoclax discontinuation remain unclear. The objective of this study was to examine real-world treatment sequence patterns post-introduction of novel agents and specifically understand treatment sequencing following venetoclax.
METHODS
The CLL Collaborative Study of Real-World Evidence (CORE) is a retrospective, multicenter, collaborative observational study of pts with CLL/small lymphocytic leukemia (SLL). For this analysis, adult pts were eligible for inclusion if they started therapy for CLL/SLL in the relapsed/refractory setting after February 12, 2014 (FDA approval date of first novel agent for CLL/SLL). Interim data are presented; data collection for this analysis is ongoing and is expected to be completed by October 31, 2019. Treatment sequences were characterized specifically focusing on treatment sequencing following venetoclax and other novel agents (i.e., BCRi: e.g., ibrutinib, idelalisib or acalabrutinib). Clinical response, as assessed by physician, was also reported from medical charts (iwCLL criteria were provided as a reference only).
RESULTS
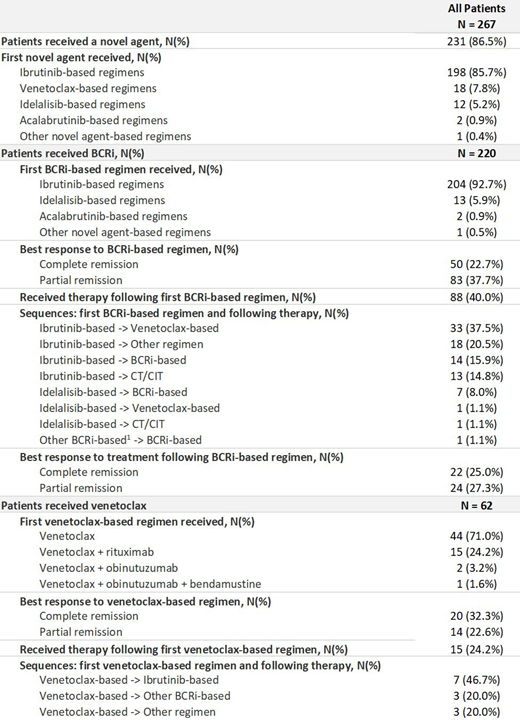
Of the 267 pts available at the time of interim data analysis, 231 pts (87%) received a novel agent in at least one line of therapy (first-line: 35 pts [15%]; second-line: 133 pts [58%]; third-line or later: 63 pts [27%]). Among novel agents, ibrutinib was the most commonly used first novel agent for 198 pts (86%) followed by venetoclax for 18 pts (8%) and idelalisib for 12 pts (5%).
Of those 267 pts, 143 pts (54%) received chemotherapy/chemoimmunotherapy (CT/CIT) followed by a novel agent; 63 pts (24%) received a novel agent followed by a novel agent; 17 (7%) received a novel agent followed by CT/CIT; and 47 (18%) received a CT/CIT followed by CT/CIT.
At the time of this interim data cut there were 220 pts (82%) who received BCRi-based regimens (first-line: 33 pts [15%]; second-line: 123 pts [56%]; third-line or later: 64 pts [29%]) and 62 pts (23%) who received venetoclax-based regimens (first-line: 2 pts [3%]; second-line: 22 pts [36%]; third-line or later: 38 pts [61%]). Of the 220 pts who received BCRi-based regimens, 50 (23%) achieved complete remission (CR) and 83 (38%) achieved partial remission (PR) (responses based on physician assessment). Of the 220 pts, 88 (40%) went on to receive a subsequent line of therapy. The most common subsequent treatments administered were venetoclax containing regimens (n= 34, 39%). Of the 88 pts who received a subsequent line of therapy after BCRi-based regimens, 22 pts (25%) achieved CR and 24 pts (27%) achieved PR.
Of the 62 pts who received venetoclax-based regimens, 20 pts (32%) achieved CR and 14 pts (23%) achieved PR. Of the 62 pts, 15 pts (24%) went on to receive a subsequent line of therapy. The most common subsequent treatment administered were BCRi containing regimens (n= 10, 67%) primarily ibrutinib-based. Of the 15 pts receiving a subsequent line of therapy after venetoclax-based regimens, 3 pts (20%) achieved CR and 4 pts (27%) achieved PR. While preliminary results are encouraging, further data collection efforts are ongoing and will be included for presentation to confirm the results and outcomes associated with different sequencing options.
CONCLUSIONS
The treatment paradigm is evolving with the introduction of novel agents. Results from this study will provide a baseline description of treatment sequence patterns enabling clinicians to benchmark the impact of the introduction of novel agents and associated outcomes. Early evidence from these results also suggests that novel agents are most commonly introduced during second line treatment and that other novel agents, including ibrutinib, following venetoclax treatment are being utilized in the real-world settings.
Mato:Pharmacyclics: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; LOXO: Consultancy, Research Funding; DTRM Biopharma: Research Funding; Johnson & Johnson: Consultancy, Research Funding; TG Therapeutics: Consultancy, Other: DSMB member , Research Funding; Acerta: Consultancy; Janssen: Consultancy; Gilead: Research Funding; AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Celgene: Consultancy. Sail:AbbVie: Employment, Other: may hold stock or stock options. Yazdy:Genentech: Research Funding; Octapharma: Consultancy; Abbvie: Consultancy; Bayer: Honoraria, Speakers Bureau. Hill:Amgen: Research Funding; Seattle Genetics: Consultancy, Honoraria; Celegene: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Research Funding; Kite: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; TG therapeutics: Research Funding; Takeda: Research Funding; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Shadman:Bigene: Research Funding; TG Therapeutics: Research Funding; AbbVIe: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Research Funding; AstraZeneca: Consultancy; Sound Biologics: Consultancy; Pharmacyclics: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Atara: Consultancy; Verastem: Consultancy; Mustang Biopharma: Research Funding; Gilead: Research Funding; Merck: Research Funding; Acerta: Research Funding; Emergent: Research Funding; Celgene: Research Funding; Sunesis: Research Funding. Manzoor:AbbVie: Employment, Other: and may hold stock or stock options. Tuncer:Abbvie: Membership on an entity's Board of Directors or advisory committees; 2018 Steering Committee: Other: reimbursement for travel to the steering committee at ASH. Allan:Janssen: Consultancy, Honoraria; Acerta Pharma: Consultancy; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC, an AbbVie company: Consultancy; Verastem Oncology, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy; Sunesis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Ujjani:PCYC: Research Funding; Pharmacyclics: Honoraria; AbbVie: Honoraria, Research Funding; Genentech: Honoraria; Atara: Consultancy; Astrazeneca: Consultancy; Gilead: Consultancy. Sharmokh:AbbVie: Employment, Other: may hold stock or stock options. Jiang:AbbVie: Employment, Other: and may hold stock or stock options. Pena:AbbVie: Employment, Other: and may hold stock or stock options. Marshall:AbbVie: Employment, Other: and may hold stock or stock options. Nielsen:AbbVie: Employment, Other: and may hold stock or stock options. Barr:Janssen: Consultancy; Astra Zeneca: Consultancy, Research Funding; Verastem: Consultancy; Genentech: Consultancy; Seattle Genetics: Consultancy; Merck: Consultancy; Celgene: Consultancy; TG Therapeutics: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie company: Consultancy, Research Funding; AbbVie: Consultancy; Gilead: Consultancy. Brown:Pfizer: Consultancy; Pharmacyclics: Consultancy; Sunesis: Consultancy; Teva: Honoraria; TG Therapeutics: Consultancy; Verastem: Consultancy, Research Funding; Sun: Research Funding; Sun Pharmaceuticals, Inc: Research Funding; Morphosys: Other: Data safety monitoring boards ; Acerta Pharma: Consultancy; AstraZeneca: Consultancy; BeiGene: Consultancy; Catapult Therapeutics: Consultancy; Dynamo Therapeutics: Consultancy; Genentech/Roche: Consultancy; Gilead: Consultancy, Research Funding; Invectys: Other: other; Janssen: Honoraria; Kite: Consultancy, Research Funding; Loxo: Consultancy, Research Funding; Novartis: Consultancy; Octapharma: Consultancy. Schuh:Pharmacyclics: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; Janssen: Speakers Bureau; Verastem: Speakers Bureau; Kite: Speakers Bureau; Gilead: Speakers Bureau; Seattle Genetics: Speakers Bureau; Jazz Pharmaceuticals: Speakers Bureau; Bristol-Myers Squibb: Research Funding. Eyre:Janssen: Honoraria, Other: travel support; Gilead: Honoraria, Other: travel support; AbbVie: Honoraria, Other: travel support. Wierda:Pharmacyclics LLC: Research Funding; Juno Therapeutics: Research Funding; Gilead Sciences: Research Funding; Miragen: Research Funding; Cyclcel: Research Funding; Oncternal Therapeutics Inc.: Research Funding; GSK/Novartis: Research Funding; Genentech: Research Funding; Xencor: Research Funding; Acerta Pharma Inc: Research Funding; KITE pharma: Research Funding; Sunesis: Research Funding; Loxo Oncology Inc.: Research Funding; Janssen: Research Funding; AbbVie: Research Funding. Skarbnik:Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Verastem Oncology: Honoraria, Research Funding, Speakers Bureau; Kite Pharma: Honoraria, Speakers Bureau; Gilead Sciences: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Acerta: Research Funding; Genentech: Honoraria, Speakers Bureau; CLL Society: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Speakers Bureau; Novartis: Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Roeker:Abbott Laboratories: Equity Ownership; AbbVie: Equity Ownership. Bannerji:Gilead: Other: travel support; Celgene: Consultancy; Celgene: Consultancy; Regeneron Pharmaceuticals, Inc.: Consultancy, Other: travel support, Research Funding; Merck: Other: travel support, Patents & Royalties: IP rights; Regeneron Pharmaceuticals, Inc.: Consultancy, Other: travel support, Research Funding; Pharmacyclics: Other: travel support; AbbVie, Inc: Consultancy; Pharmacyclics: Other: travel support; Gilead: Other: travel support; AbbVie, Inc: Consultancy, travel support; Merck: Other: travel support, Patents & Royalties: IP rights. Pauff:AbbVie Inc: Employment, Other: may own stock or stock options. Schuster:Novartis, Nordic Nanovector, and Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: a patent (with royalties paid to Novartis) on combination therapies of CAR and PD-1 inhibitors.; Novartis, Celgene, Genentech, Merck, Pharmacyclics, Acerta, and Gilead: Other: Grants, Research Funding; Nordic Nanovector, Pfizer, AstraZeneca, Loxo Oncology, Acerta, and Celgene: Honoraria. Follows:Roche: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau. Cheson:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Symbios: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trillium: Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite: Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees; Acerta: Consultancy, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Research Funding; Bristol Myers Squibb: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Portola: Research Funding; Gilead: Research Funding; Epizyme: Research Funding. Eichhorst:Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BeiGene: Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; ArQule: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Brander:Genentech: Consultancy, Honoraria, Research Funding; DTRM Biopharma: Research Funding; TG Therapeutics: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Research Funding; BeiGene: Research Funding; Novartis: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy; AbbVie: Consultancy, Honoraria, Research Funding; Acerta: Research Funding; Tolero: Research Funding; Teva: Consultancy, Honoraria; MEI: Research Funding. Pivneva:AbbVie: Other: employee of Analysis Group, Inc., which has received consultancy fees from AbbVie. Lamanna:AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Verastem: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Bei-Gene: Research Funding; TG Therapeutics: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Roche-Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal