Introduction: Patients (pts) with relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL) who failed treatment with the Bruton's tyrosine kinase inhibitor (BTKi) ibrutinib have a poor outcome and are difficult to treat. This ongoing, two-cohort, Phase II trial evaluates the safety and preliminary efficacy of tafasitamab (MOR208), an Fc-enhanced anti-CD19 monoclonal antibody in combination with idelalisib (IDE) (Cohort A) or venetoclax (VEN) (Cohort B) in R/R CLL pts previously treated with a BTKi. Preliminary results were published at EHA 2018 for Cohort A and at ASH 2018 for Cohort B. Here, we report the results of the primary analysis for both cohorts.
Methods and Patients: Pts who either progressed or were intolerant to BTKi were enrolled at 12 sites in six countries in Europe and the US from Nov 2016 to Apr 2018. The primary endpoint is the incidence and severity of adverse events (AEs); secondary endpoints include overall response rate (ORR) as per investigator assessment according to International Workshop on CLL (IWCLL) 2008 guidelines. Complete response (CR) was confirmed by computed tomography assessment and by bone marrow (BM) biopsy. The exploratory endpoint minimal residual disease (MRD) was assessed centrally by quantitative allele-specific oligonucleotide-polymerase chain reaction (ASO-PCR) in peripheral blood (PB) and BM. Each treatment cycle (C) lasts 28 days (D). Dose and administration: tafasitamab intravenous infusion, 12 mg/kg weekly in C1-C3, every other week in C4-C6 and monthly from C7D1; IDE orally, 150 mg twice daily; VEN orally, weekly ramp up starting on C1D8 at 20 mg to full daily dose of 400 mg. Patients: mean time since first CLL diagnosis was 135 months (mos) for pts in Cohort A and 105 mos in Cohort B. Median number of prior therapy lines was five (2-9) and three (1-5), respectively. All pts had received ibrutinib; one pt had subsequent acalabrutinib treatment as last prior therapy line. Mutations of BTK and PLCγ2 were assessed in nine pts in Cohort A and 13 pts in Cohort B. BTK/PLCγ2 mutations were centrally detected in 4/3 pts in Cohort A and in 2/3 pts in Cohort B, respectively. Complex karyotype was observed in six (54.5%) pts in Cohort A and 12 (92.3%) pts in Cohort B. Results with a data cut-off date of 9 Nov 2018 are presented.
Results:
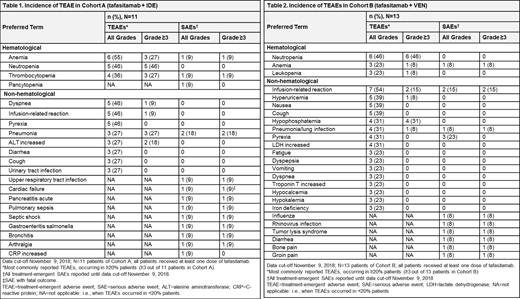
Cohort A: Median time on study was 9.9 mos (95% confidence interval [CI]: 5.7-not reached). Eleven pts were enrolled and received tafasitamab and IDE. Two pts discontinued treatment due to AEs (aspartate-aminotransferase increased; acute pancreatitis), two due to progressive disease (PD) and one pt by physician's decision. One pt died due to PD and one pt due to cardiac failure. At the cut-off date, treatment was ongoing in four pts. Table 1 summarizes treatment-emergent adverse events (TEAEs) with neutropenia Grade ≥3 being most common (5 [46%]). Fourteen treatment-emergent serious AEs (SAEs) were reported in eight (72.7%) pts. ORR was 90.9% (CR=9.1%, partial response [PR]=81.8%), disease control was achieved in all 11 pts. One of eight pts (12.5%) assessed for MRD status reached MRD-negativity in PB at C14.
Cohort B: Median time on study was 12 mos (95% CI: 2.8-not reached). Eleven of 13 enrolled pts received tafasitamab and VEN while two pts received tafasitamab only. Three pts discontinued treatment due to AEs (infusion-related reactions [two pts], diarrhea [one pt], one due to PD and one withdrew consent. At the cut-off date, treatment was ongoing in eight patients. Table 2 summarizes TEAEs, neutropenia Grade ≥3 was most commonly observed (six [46%] pts). Fourteen SAEs were reported in nine (69.2%) pts. The ORR in all 13 pts was 76.9% (CR=23.1%, PR=53.8%, not evaluable=23.1%). Six of seven pts assessed for MRD in PB (46.2% of 13 [100%] pts) reached negative status in PB by C7 at the latest. One of three pts assessed for MRD in BM (7.7% of 13 [100%] pts) reached MRD negative status in BM at C15.
Conclusions: This trial demonstrates that in heavily pretreated pts with R/R CLL who failed prior BTKi, tafasitamab in combination with IDE or VEN is a potential therapeutic option. The safety profiles of the combinations are influenced by the combination partner, but both combinations are manageable. The response rates and MRD-negativity rates indicate that combinations of targeted agents with anti-CD19 tafasitamab have valuable antitumor activity and warrant further investigation of tafasitamab-based combinations in CLL.
Staber:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Honoraria, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda-Millenium: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Jurczak:Celtrion: Research Funding; Sandoz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Research Funding; Celgene Corporation: Research Funding; Incyte: Research Funding; Servier: Research Funding; Roche: Research Funding; Novo Nordisk: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Research Funding; Gilead: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Loxo: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding. Brugger:AstraZeneca: Equity Ownership; MorphoSys: Employment. Chanan-Khan:Pharmacyclics: Research Funding; Merck: Research Funding; Jansen: Research Funding; Mayo Clinic: Employment; Ascentage: Research Funding; Millennium: Research Funding; Xencor: Research Funding; AbbVie: Research Funding. Greil:Mundipharma: Honoraria, Research Funding; Bristol-Myers-Squibb: Consultancy, Honoraria, Other: Travel/accomodation expenses, Research Funding; Daiichi Sankyo: Consultancy, Honoraria; Sanofi Aventis: Honoraria; Sandoz: Honoraria; AstraZeneca: Consultancy, Honoraria, Other: Travel/accomodation expenses, Research Funding; Celgene: Consultancy, Honoraria, Other: Travel/accomodation expenses, Research Funding; Eisai: Honoraria; Janssen-Cilag: Honoraria; Cephalon: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Other: Travel/accomodation expenses, Research Funding; Boehringer Ingelheim: Honoraria; Amgen: Consultancy, Honoraria, Other: Travel/accomodation expenses, Research Funding; Ratiopharm: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; MSD: Consultancy, Honoraria, Other: Travel/accomodation expenses, Research Funding; Pfizer: Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Other: Travel/accomodation expenses, Research Funding; Roche: Consultancy, Honoraria, Other: Travel/accomodation expenses, Research Funding; GSK: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Dirnberger-Hertweck:MorphoSys: Employment. Kelemen:MorphoSys: Employment. Middeke:Janssen: Consultancy, Speakers Bureau; MSD: Consultancy; AbbVie: Consultancy, Speakers Bureau; Gilead: Consultancy; Roche: Speakers Bureau; Sanofi: Research Funding, Speakers Bureau. Montillo:Roche: Consultancy, Honoraria, Research Funding; Acerta: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Speakers Bureau; Versatem: Membership on an entity's Board of Directors or advisory committees. Munir:Acerta: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Other: TBC; AbbVie: Honoraria; Alexion: Honoraria; Gilead: Honoraria; Janssen: Honoraria; Novartis: Honoraria; Roche: Honoraria; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sunesis: Consultancy. Parikh:Pharmacyclics: Honoraria, Research Funding; Ascentage Pharma: Research Funding; Genentech: Honoraria; MorphoSys: Research Funding; Acerta Pharma: Research Funding; AbbVie: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Janssen: Research Funding. Stilgenbauer:Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; GSK: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Hoffmann La-Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau. Weirather:MorphoSys: Employment. Woyach:Janssen: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; AbbVie: Research Funding; Karyopharm: Research Funding; Loxo: Research Funding; Morphosys: Research Funding; Verastem: Research Funding. Wendtner:AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Hoffman-La Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead Sciences, Inc.: Consultancy, Honoraria, Research Funding, Speakers Bureau; MorphoSys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal