Atypical chronic myeloid leukemia (aCML) is a rare BCR-ABL1 negative clonal disorder, which belongs to the myelodysplastic/myeloproliferative group. This disease is characterized by recurrent somatic mutations in several genes including SETBP1, ASXL1 and ETNK1, as well as high genetic heterogeneity, thus posing a great therapeutic challenge. The clinical prognosis for aCML is poor, with a median overall survival of 18 months after diagnosis, and no established standards of care exist for its treatment. The dissection of the molecular processes underlying aCML leukemogenesis could therefore result decisive in ameliorating the prognosis for aCML.
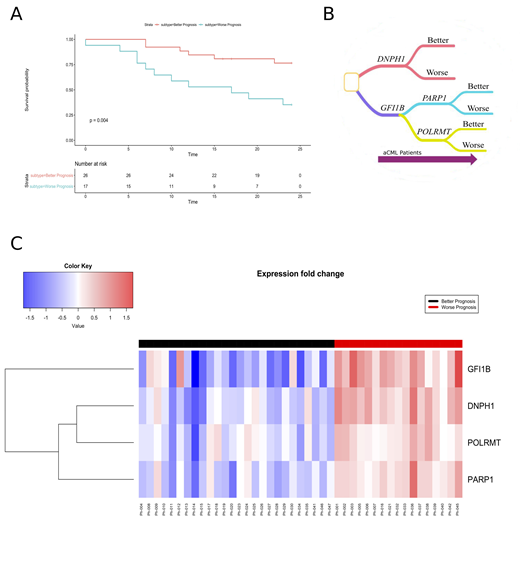
With the aim to provide a comprehensive genomic characterization of aCML and to link the detected alterations with the clinical course of the disease, we applied a high-throughput sequencing strategy to 43 aCML samples, including whole-exome sequencing and RNA sequencing. Our study confirms ASXL1 and SETBP1 as the most frequently mutated genes with a total of 43.2% and 30.2%, respectively; ETNK1 mutations are observed in 14% of patients. An average of 2 mutations per patient was observed [range: 0-5]. We characterized the clonal architecture in a subset of 8 aCML patients by means of colony assays and targeted resequencing. The results indicate that ETNK1 variants occur very early in the clonal evolution history of aCML, while SETBP1 mutations represent a late event; interestingly, in the two cases where ASXL1 was mutated together with SETBP1, its mutations occupied an intermediate hierarchical position. CBL mutations, when present, showed a tendency toward reaching homozygosity through somatic uniparental disomy. Stratification based on RNA-sequencing gene expression data (Ramazzotti, Daniele, et al. Nature communications 9.1 (2018): 4453) identified two clearly different populations (26 and 17 patients) in terms of Overall Survival (OS), with 2 year OS of 69.23% [95% IC: 48.21%-86.67%] and 35.29% [95% IC: 14.21%-61.67%] respectively (logrank test for trend: p=0.004, Fig. 1A). In addition, the group with better prognosis showed a higher frequency of ETNK1 mutations (hypergeometric test: p=0.032). We next performed differential gene expression analysis to detect genes differentially expressed between the two patients' populations. This analysis revealed 38 significant genes (t-test p-value adjusted for false discovery rate p<0.01) overexpressed in the group with negative outcome. Notably, the majority of these genes are known cancer drivers, such as IDH2, MEN1, MYC and TP53. Involved pathways include gene transcription and cell differentiation, mitochondrial activity and DNA repair. We then considered RNA-sequencing data for the 4 most significant genes within the previous list (namely DNPH1, GFI1B, PARP1 and POLRMT) to build a classifier capable of associating patients to the respective subtype (better vs. worse prognosis). Our results show that a random forest classifier (Ho, Tin Kam. Proceedings of 3rd international conference on document analysis and recognition. Vol. 1. IEEE, 1995) using the 4 most significant genes achieves a 93.79% accuracy assessed by means of 10 fold cross validation (Fig.1B-C).
In conclusion, we present here the first description of a large aCML cohort, in which sequencing data, clonal hierarchy of mutations and gene expression profiles were integrated through bioinformatics analysis. RNA-sequencing data stratification characterizes two groups with different prognosis; a classifier based on the 4 top differently expressed genes accurately predicts patients' outcome.
Figure 1. A) Overall Survival curve (Kaplan-Meier curve) at 24 months shows significant different outcomes (p=0.004). B) Random forest classifiers learn multiple decision trees in order to predict outcomes. In the figure, an example of decision tree where nodes are genes and leaves are outcomes (better/worse prognosis). C) Heatmap of expression fold change for the top four differentially expressed genes.
Rea:BMS: Honoraria; Incyte Biosciences: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Stagno:Pfizer: Honoraria; BMS: Honoraria; Incyte: Honoraria; Novartis: Honoraria. Elli:Novartis: Membership on an entity's Board of Directors or advisory committees. Gambacorti-Passerini:Pfizer: Honoraria, Research Funding; Bristol-Meyers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal