Introduction: Anemia is the most frequent cytopenia in myelodysplastic syndromes (MDS) and patients will require red blood cell transfusion. Most low-risk MDS patients with anemia are currently treated with erythropoietin stimulating agents (ESA). This treatment gives 45-60% of erythroid response but responders will lose their erythroid response after a median time of 20-24 months (Park, Blood, 2008). Recently, luspatercept (RAP-536), a trap ligand of transforming growth factor-β superfamily, including activins and growth differentiation factors (GDFs), has shown positive effects on erythroid response in MDS patients especially in SF3B1 mutated patients (Platzbecker, Lancet Oncology, 2017). The objective of our work was to evaluate the effect of RAP-536 on human engraftment in PDX mice models of MDS.
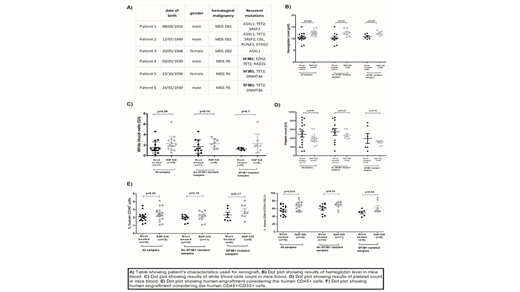
Methods: We performed patient derived xenograft (PDX) NOD SCID gamma (NSG) murine model by intrabone injections of mesenchymal stromal cells (MSC) and CD34+ hematopoietic stem progenitor cells (HSPC) from 3 MDS patients with SF3B1 mutations and 3 MDS patients without SF3B1 mutations but carrying other recurrent mutations (ASXL1, TET2, SRSF2, CBL, RUNX1, STAG2) seen in MDS (Table A). Three months after xenograft, we have realized a bone marrow aspiration for monitoring human engraftment using flow cytometry to check that there was a human engraftment. Then, the mice were treated for 2 months with 30mg/kg/week of RAP-536. At the end of the procedure, serum, blood and bone marrow were collected.
Results: As GDF11 is a target of RAP-536, we have measured GDF11 level in serum in order to confirm the expected effects of RAP-536. We observed decreased levels of GDF11 in RAP-536 treated mice versus mock treated (p<0.0001). Concerning blood counts, we observed a higher level of hemoglobin (p=0.002) (Fig. B) and a higher level of white blood cells (p=0.04) (Fig. C) in RAP-536 treated mice, independently of the SF3B1 mutated status and no impact on platelet count (Fig. D). Human myeloid engraftment was determined by flow cytometry. If we consider human DAPI-/murine CD45-/ human CD45+ positive cells, the average percentage was 2.09% for mock treated mice and 2.4% for treated mice (no statistically significant difference between treated mice or not (p=0.09) and no impact of SF3B1 mutated status) (Fig. E). Interestingly, if we focused on human CD45+/CD33+ among the human CD45+ cells, we observed a higher engraftment 66.35% of positive cells versus 55.89% in treated mice vs mocked treated mice respectively (p=0.014), independently of SF3B1 mutated status (Fig. F). Molecular analyzes on CD45+ cells harvested from the PDX model are ongoing to confirm the human engraftment by detecting the recurrent gene mutations present in the initial MDS clones. Mutation burden level will be analyzed in the presence or absence of RAP-536.
Conclusion: RAP-536 improves hemoglobin and WBC levels and seems to enhance the engraftment of human myeloid hematopoiesis in this PDX murine model of MDS. Molecular analyses are ongoing to clarify the relative proportions of normal and pathological human hematopoiesis before and after RAP-536.
Park:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding.
RAP-536
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal