One of the most common secondary resistance mechanisms to ibrutinib (IBR, a first-generation, irreversible Bruton's tyrosine kinase [BTK] inhibitor) in CLL is the development of mutations in BTK involving Cys481, leading to impaired drug binding (Woyach et al, NEJM 2014; Furman et al, NEJM 2014). The mechanisms of resistance to second generation BTK inhibitors are currently unknown. We aimed to assess the spectrum of acquired BTK mutations in patients with CLL progression on zanubrutinib (ZANU), a second-generation, irreversible inhibitor of BTK.
We identified 38 CLL patients, treated with ZANU on clinical trials (NCT02343120, NCT02569476, NCT03336333, NCT02795182) at three centres, for whom serial samples were available. Four of 38 patients (10.5%) had CLL progression on ZANU (time to progression 5, 26, 29 and 48 months) and underwent amplicon next generation sequencing (NGS) of BTK (exon 11, 15, 16) and PLCG2 (exon 16, 19-20, 24, 27-28). Remarkably, we detected a BTK kinase domain mutation, BTK Leu528Trp (NM_000061.2:c.1583T>G), in all four patients progressing on ZANU. In addition, all four patients had detectable Cys481 mutations at lower variant allele frequency (VAF) than the BTK Leu528Trp (median BTK Leu528Trp 34.9% vs BTK Cys481 9.1%). Analysis of sequence reads from amplicon NGS and RNA-sequencing data demonstrated that BTK Leu528Trp and BTK Cys481 mutations were present on different alleles. Assessment of the BTK Leu528Trp and BTK Cys481 mutations with high sensitivity droplet digital PCR (ddPCR) confirmed the absence of both mutations prior to ZANU exposure in all patients (sensitivity 0.1% VAF). Longitudinal analysis of the four patients with the BTK Leu528Trp mutation demonstrated the appearance of the Leu528Trp coincident with rising measurable disease and subsequent clinical CLL progression. We then went on to test patients on ZANU without disease progression but with persistent measurable disease (n=34) by ddPCR and detected three further patients harbouring low level BTK Leu528Trp mutations (VAF <1%). These mutations were first detected after a median of 40 months on ZANU therapy.
The BTK Leu528Trp mutation has been described only once previously in a patient in the context of IBR resistance (who transformed with Richter's syndrome) where it co-occurred with Cys481 mutations (Maddocks et al, JAMA Oncol 2015). As the prevalence of BTK Leu528Trp among progressive disease samples in our cohort exceeds all prior reports in IBR-treated patients, we sought to further understand the specificity of BTK Leu528Trp for ZANU progression. Targeted sequencing in a cohort of 49 patients progressing on IBR from the European Research Initiative on CLL (ERIC) did not detect the BTK Leu528Trp in any patients (sensitivity 1% VAF).
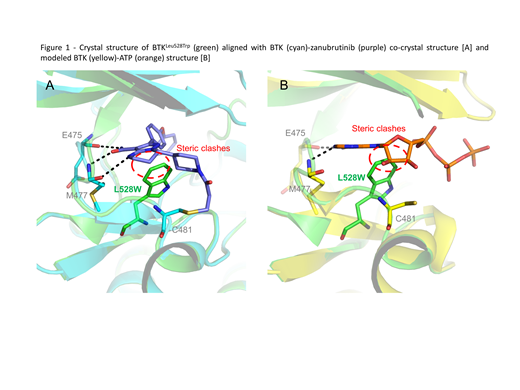
We went on to perform biochemical and cellular studies on the BTK Leu528Trp mutation. Assessment of enzymatic activity of BTKLeu528Trp demonstrated a significant loss of activity compared to both BTKWT and BTKCys481Ser. This was further confirmed by assessing BTK autophosphorylation in HEK293 cells. Autophosphorylation at BTK Tyr223 was markedly reduced in HEK293 cells stably expressing BTKLeu528Trp compared to both BTKCys481Ser and BTKWT. In addition, a crystal structure of apo-BTKLeu528Trp was solved to understand effects of BTKLeu528Trp on ZANU binding to BTK. The alignment of the crystal structure of apo-BTKLeu528Trp with that of BTKWT-ZANU or the modeled structure of BTK-ATP suggested potential steric clashes between BTKLeu528Trp and ZANU (Figure 1A), as well as BTKLeu528Trp and ATP (Figure 1B).
In conclusion, we have described the novel enrichment of BTK Leu528Trp mutations occurring in patients with CLL progressing on ZANU and both structural and experimental data consistent with this mutation resulting in interference with both ATP and ZANU binding to BTK. These findings emphasize the potential for agent-specific resistance mutations with second generation BTK inhibitors and the need to include these mutations in diagnostic screening for BTK resistance in the clinic.
SH/CPST co-first authors, CT/PB co-senior authors
Handunnetti:Abbvie: Other: Travel Grant; Gilead: Honoraria. Zhou:Beigene: Employment. Sun:Beigene: Employment. Xing:Beigene: Employment. Zhang:Beigene: Employment. Guo:Beigene: Employment. Sutton:Abbvie: Honoraria; Gilead: Honoraria; Janssen: Honoraria. Ghia:Dynamo: Consultancy, Honoraria; ArQule: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Sunesis: Consultancy, Honoraria, Research Funding; Acerta/AstraZeneca: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie Company: Consultancy; Novartis: Research Funding; Juno/Celgene: Consultancy, Honoraria. Scarfo:AstraZeneca: Honoraria; Janssen: Honoraria; AbbVie: Honoraria. Seymour:Takeda: Consultancy; Acerta: Consultancy; Celgene: Consultancy, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding; Roche: Consultancy, Research Funding, Speakers Bureau. Anderson:Walter and Eliza Hall Institute: Employment, Patents & Royalties: Institute receives royalties for venetoclax, and I receive a fraction of these.. Roberts:AbbVie: Other: Unremunerated speaker for AbbVie, Research Funding; Australasian Leukaemia and Lymphoma Group: Membership on an entity's Board of Directors or advisory committees; Walter and Eliza Hall Institute: Patents & Royalties: Institute receives royalties for venetoclax, and I receive a fraction of these.; Janssen: Research Funding; BeiGene: Research Funding. Huang:Genentech: Patents & Royalties: DCSH is an employee of the Walter and Eliza Hall Institute which receives milestone and royalty payments related to venetoclax. Liu:Beigene: Employment. Cheah:Roche, Janssen, MSD, Gilead, Loxo Oncology, Acerta, BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene, Roche, Abbvie: Research Funding; Roche: Other: Travel expenses. Tam:Janssen: Honoraria, Research Funding; BeiGene: Honoraria; Pharmacyclics LLC, an AbbVie company: Honoraria; Roche: Honoraria; Novartis: Honoraria; AbbVie: Honoraria, Research Funding. Blombery:Janssen: Honoraria; Invivoscribe: Honoraria; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal