Introduction:
Interferon alpha (IFNa) based therapies can induce sustained hematological and molecular responses (HR/MR) in Polycythemia Vera (PV) and other Myeloproliferative Neoplasms (MPN), however, not all patients respond sufficiently. While previous studies suggest that disease driving somatic mutations and genomic aberrations do not predict response to IFNa, the role of common germline polymorphisms remains elusive. We addressed the effect of germline genetic factors on PV therapy with Ropeginterferon alfa-2b (Ropeg), a novel monopegylated IFNa. We performed genome-wide association studies (GWAS) as an unbiased approach, and additionally evaluated a previously reported influence of polymorphisms at the IFNL4 locus in a large PV patient cohort.
Methods:
Genomic DNA was isolated from whole blood of PV patients (n=115) on Ropeg therapy, who provided consent within the PROUD-PV (NCT01949805) and CONTI-PV (NCT02218047) study programs. Patients were genotyped for >900k tagging single nucleotide polymorphisms (SNPs) across the genome on the Affymetrix SNP 6.0 array platform. Additional IFNL4 SNPs were typed using an amplicon-based next generation sequencing approach. Association analyses were performed using the PLINK toolset. JAK2-V617F mutant allele burden was quantified using a qPCR-based assay (ipsogen MutaQuant, Qiagen). MR was measured based on changes in mutant allele burden upon therapy as defined by the European LeukemiaNet (ELN) criteria.
Results:
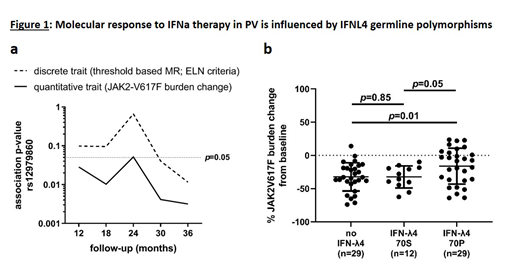
To test for potential associations between germline genetic variation and response to IFNa therapy, we performed GWAS for MR data upon 12 months (M), 18M, 24M and 30M and 36M follow-up on Ropeg treatment. Genome-wide tagging SNPs were tested both for association in a case-control setup applying chi-square statistics as well as for quantitative trait association, using peripheral blood JAK2-V617F mutational burden changes from baseline as continuous variables. After Bonferroni correction for multiple testing, none of those analyses revealed a statistically significant association, suggesting the absence of strong germline predisposition factors for MR. Germline variation at the interferon-lambda (IFNL) locus was previously reported to strongly influence viral clearance during IFNa therapy of Hepatitis C (reviewed in Wack et al., Nat Immunol, 2015). While the same variants were recently reported to also affect HR during IFNa treatment in an MPN patient cohort (Lindgren et al., EJH, 2018), their potential impact on MR as surrogate marker for the size of the malignant MPN clone has not yet been evaluated. Upon testing for MR in our PV cohort, in a case-control setup we observed a statistically significant association only upon 36M follow-up (p=0.02; OR=2.51; 95%CI=[1.14-5.62]; Figure 1a). Notably, testing for change of JAK2-V617F mutational burden under Ropeg treatment as quantitative trait, we found the association to be present at formal statistical significance at all stages during follow-up (Figure 1a). For the Hepatitis C association, it is now widely acknowledged that a diplotype of two coding variants covers most of the causality, where rs368234815_TT disrupts the open reading frame (no IFNL4) while rs117648444_G results in the impaired IFNL4-S70 in contrast to the fully functional IFNL4-P70, the latter paradoxically impacting negatively on viral clearance (Terczynska-Dyla et al., Nat Commun, 2014). Similarly, in our PV cohort genotyping and phasing of this diplotype revealed a significant influence on MR in carriers of the fully functional IFNL4-P70 (p=0.01; Figure 1b; shown are patients at 36M follow-up (n=70)).
Conclusions:
The absence of a strong germline predisposition factor for Ropeg treatment response in our cohort implies that any PV patient may be eligible for Ropeg therapy independently of their genetic makeup. While the IFNL4 diplotype is of potential use for patient stratification, it remains to be investigated whether increase of treatment duration and/or dose adjustments can overcome the adverse effect of functional IFNL4 on Ropeg therapy. Longitudinal monitoring of JAK2-V617F mutational burden under Ropeg treatment in conjunction with determination of the IFNL4 germline genetic status may allow for optimizing patient management.
Krejcy:AOP Orphan Pharmaceuticals AG: Employment. Klade:AOP Orphan Pharmaceuticals AG: Employment. Zoerer:AOP Orphan Pharmaceuticals AG: Employment. Gisslinger:Janssen-Cilag: Honoraria; Roche Austria GmbH: Consultancy; Novartis Pharma GmbH: Consultancy, Honoraria, Research Funding; Myelopro GmbH: Consultancy; Celgene GmbH: Honoraria; Pharma Essentia: Other: Personal fees; AOP Orphan Pharmaceuticals: Consultancy, Honoraria, Research Funding. Kralovics:Novartis: Honoraria; AOP Orphan Pharmaceuticals AG: Honoraria, Other: Advisory board; Pharma Essentia: Honoraria; Qiagen: Honoraria; MyeloPro Diagnostics and Research: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal