Introduction: The presence of a molecular driver mutation such as JAK2 V617F is a major criterion for diagnosing myeloproliferative neoplasms. JAK2 V617F allelic burden (percentage of cells harboring the mutation) has been associated with varying disease phenotypes and outcomes. Myelofibrosis (MF) with low allelic burden is associated with a myelodepletive phenotype, which includes shorter overall survival, anemia, leukopenia, and lesser degree of splenomegaly. Some JAK inhibitors utilized in patients with low allelic burden (<50%) may achieve smaller spleen volume reduction (SVR), therefore, treatment of the lower allelic burden population remains clinically challenging. Pacritinib is an oral JAK2/IRAK1 inhibitor with demonstrated efficacy based on SVR from two phase 3 studies in MF patients including those with severe thrombocytopenia (PERSIST-1 [P1] and PERSIST-2 [P2]). These studies were unique in that the combined P1/P2 median allelic burden is 47% as compared to other phase 3 studies of JAK2 inhibitors with a median allelic burden >80%. To better evaluate the impact of allelic burden on pacritinib-induced SVR in MF patients, a retrospective analysis was performed on the combined data from the P1 and P2 studies.
Methods: Patients ≥18 years of age with primary or secondary MF, palpable splenomegaly ≥5 cm below the left costal margin, and DIPSS risk category of intermediate-1, intermediate-2, or high-risk were eligible for the studies. Patients with any baseline platelet count were eligible for P1, while P2 included only those with baseline counts ≤100,000/μL. Prior JAK inhibitor therapy was allowed in P2, but not P1. Patients in P1 received pacritinib 400 mg once daily (QD) or best available therapy (BAT); those in P2 received pacritinib 400 mg QD, pacritinib 200 mg twice daily (BID), or BAT. In both studies, BAT consisted of physician-selected treatments for MF (including symptom-directed treatment or watch and wait); treatment with ruxolitinib was allowed as BAT only in P2. The percentage of patients achieving ≥35% SVR at week 24 was a primary endpoint for both studies. This retrospective analysis assessed those patients with JAK2 V617F for possible relationship between allelic burden and SVR at 24 weeks.
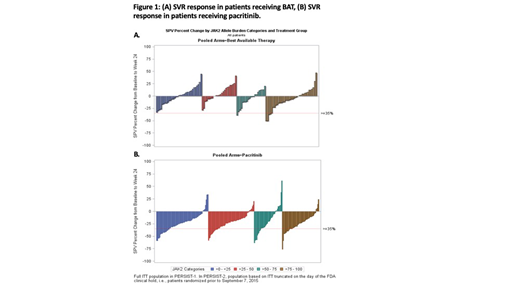
Results: JAK2 V617F allelic burden data were available for 363 patients that received pacritinib and 173 patients that received BAT. 19.6% of BAT subjects received ruxolitinib during study. Pacritinib demonstrated similar SVR across all allelic burden quartiles, including the lowest quartile. In contrast, SVR responses with BAT were more frequently observed in those patients with higher allelic burden (>75-100% quartile; Figure 1). The percentage of patients with ≥35% SVR was significantly higher with pacritinib compared with BAT for those with an allele burden ≤50% (allele burden >0-25%: pacritinib=18/86 [21%], BAT=0/46, P<0.001; allele burden >25-50%: pacritinib=14/91 [15%], BAT=0/33, P=0.020); there was no significant difference in the >50-75% (pacritinib=9/53 [17%], BAT=1/26 [4%], P=0.153) and >75-100% quartiles (pacritinib=14/72 [20%], BAT=5/49 [10%], P=0.209).
Conclusions: Myelofibrosis (MF) with JAK2 V617F allelic burden <50% is associated with a myelodepletive phenotype and a worse prognosis. MF patients with low allelic burden may have a non-JAK mediated disease which could explain why other JAK inhibitors have been shown to be less effective in patients with low allelic burden. Pacritinib provides similar SVR across all levels of allelic burden, including levels <50% unlike BAT (which included ruxolitinib). This data suggests that pacritinib may provide benefit over a wider range of patients with MF compared to other JAK inhibitors.
Verstovsek:Incyte: Research Funding; Roche: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding; Gilead: Research Funding; Promedior: Research Funding; CTI BioPharma Corp: Research Funding; Genetech: Research Funding; Blueprint Medicines Corp: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Research Funding; Pharma Essentia: Research Funding; Astrazeneca: Research Funding; Ital Pharma: Research Funding; Protaganist Therapeutics: Research Funding; Constellation: Consultancy; Pragmatist: Consultancy. Scott:Celgene: Consultancy; Novartis: Consultancy; Agios: Consultancy; Incyte: Consultancy. Taylor:Baxalta: Research Funding; CTI BioPharma: Employment, Equity Ownership. Mascarenhas:Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Research Funding; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Roche: Consultancy, Research Funding; Merck: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Promedior: Research Funding; Merus: Research Funding; Pharmaessentia: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal