Introduction
Current treatment options for myelofibrosis (MF) are diverse; apart from rare patients who are eligible for allogeneic transplantation, active treatments are focused on controlling MF-related symptoms and are not disease modifying. Patients who are initially asymptomatic and those at low risk for progression are usually observed without active treatment (watch and wait) until the appearance of symptoms or progression. There are limited real-world data on the management of patients with MF in the United Kingdom (UK). The REALISM UK study documented the early management pathways for patients with MF in routine clinical practice.
Method
REALISM UK was a multi-centre, retrospective, non-interventional study in 15 UK secondary care centres. Eligible patients were those aged ≥18 years at diagnosis of MF, with diagnosis >6 months and ≤5 years prior to data collection and with ≥1 follow-up visit. Data on patient demographics, clinical characteristics, MF management strategies and outcomes were collected from patients' medical records; the observation period was from the date of MF diagnosis until data collection. The primary endpoint was the time from diagnosis to active treatment. Watch and wait strategies included supportive therapies (blood transfusions, erythropoiesis-stimulating agents, and steroids).
Results
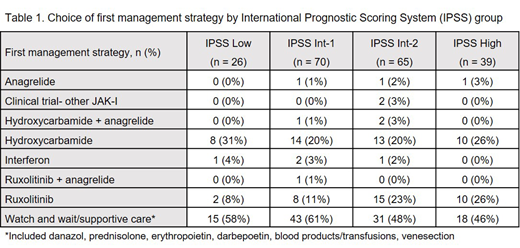
A total of 200 patients were recruited (63% [n=126/200] with primary MF (PMF); 37% [n=74/200] with secondary MF). The median age at MF diagnosis was 69.7 years (interquartile range [IQR] 63.5-75.7) and 71% (n=141/200) had a JAK2 mutation. At diagnosis, 52% (n=104/200) of patients had International Prognostic Scoring System (IPSS) categories of intermediate-2 (Int-2) or high. Common documented features of disease at diagnosis included an enlarged spleen (47%, n=94/200), anaemia (44%, n=88/200), and constitutional symptoms (e.g. night sweats, unexplained fever, or weight loss [30%, n=60/200]). The median (IQR) time to first active treatment was 46 days (range 0-350) in the total population; patients with higher risk disease were prescribed active treatment sooner: IPSS low, 136.5 days (range 0-625; n=26); IPSS Int-1, 90 days (range 0-494; n=70); IPSS Int-2, 0 days (range 0-252; n=65); IPSS high: 0 days (0-216; n=39). The choice of first management strategy by IPSS at diagnosis is shown in Table 1. During the study period, watch and wait was the first management strategy for 54% (n=107/200) of patients, with the median (IQR) time from diagnosis to active treatment in this group being 322 (130-610) days. Constitutional symptoms were recorded in 20% (n=21/107) of patients for whom watch and wait was the first management strategy. Active treatment was started at diagnosis for 47% (n=93/200) of patients, with the most commonly used medications being hydroxycarbamide (22.5% [n=45/200]) and ruxolitinib (17.5% [n=35/200]). The median (IQR) treatment duration during the study observation window was 346 days (range 121-768) for hydroxycarbamide and 375 days (range 172-691) for ruxolitinib. In total, 5.5% (n=11/200) of patients were recorded as having undergone allogenic stem cell transplantation during the observation period.
Conclusion
Clinicians in the UK apply a broad range of management strategies for the treatment of patients with MF. Many patients were observed without active treatment following diagnosis, despite having symptomatic disease. The most common active treatments as first management strategies were hydroxycarbamide and ruxolitinib.
Mead:Pfizer: Consultancy; Novartis: Consultancy, Honoraria, Other: Travel/accommodation expenses, Research Funding, Speakers Bureau; Bristol Myers-Squibb: Consultancy. Somervaille:Novartis: Consultancy, Honoraria. Butt:Novartis: Consultancy, Honoraria, Speakers Bureau. Whiteway:Novartis: Consultancy, Honoraria, Speakers Bureau. Kirkpatrick:Novartis: Consultancy, Honoraria. Roughley:Novartis: Consultancy, Honoraria. Ewing:Novartis: Honoraria, Other: Meeting attendance sponsorship ; Bristol Myers-Squibb: Other: Meeting attendance sponsorship . Garg:Novartis: Consultancy, Honoraria. Harrison:Promedior: Honoraria; Shire: Speakers Bureau; Sierra Oncology: Honoraria; Celgene: Honoraria, Speakers Bureau; Roche: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau; Incyte: Speakers Bureau; Gilead: Speakers Bureau; CTI: Speakers Bureau; AOP: Honoraria; Janssen: Speakers Bureau. Bains:Novartis: Employment. Chiu:Novartis: Employment. Hickey:OPEN VIE: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal