The risk of HBVr in chronic myeloid leukemia (CML) has been reported in many studies but there are no clear guidelines or recommendation regarding screening and monitoring of HBV in CML patients receiving tyrosine kinase inhibitors (TKIs).
This review addresses the following questions regarding screening, monitoring, prophylaxis and treatment of HBVr in CML patients receiving TKIs:
Who should be screened before starting TKIs?
Which type of screening test should be used?
Should HBV DNA be used to screen or monitor the patients before starting TKI?
What is the best timeline to monitor HBV reactivation?
METHODS:
Studies from the year 2000 to date were searched on PubMed and Google Scholar, which investigated the possibility of HBV reactivation on patients with CML treated with TKIs. 11 studies were selected and reviewed.
All patients are above 18 years old, diagnosed with CML on TKI treatment, were included in this review. Patients with any hepatitis serology marker at baseline (upon diagnosis of CML) were also included. Patients with active hepatitis B infection before the start of TKIs were excluded from the review.
RESULTS:
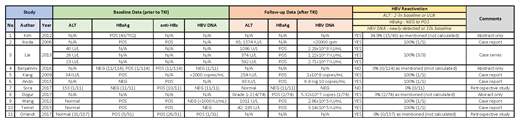
We identified 25 reported cases of hepatitis B reactivation after TKI treatment. 22/25 had HBsAg positive at baseline (before starting TKI) and 1/25 had HBsAg negative at baseline. The remaining 2/25 had reactivation with no clear baseline test results. These results are illustrated in Table 1.
DISCUSSION:
HBVr is defined by changes in (ALT) levels, HBV serological markers, or HBV DNA. Thus, HBVr is considered when:
A 2- to 3-fold increase of ALT above baseline or the upper limit of normal.
A reverse hepatitis B surface antigen (HBsAg) seroconversion or detection of HBV DNA in the blood in the absence of HBsAg.
A newly detected HBV DNA or a ≥10-fold (1log10) rise in HBV DNA level.
The risk of HBVr in patients receiving tumor necrosis factors (TNF), moderate-to-high dose steroids for 4 weeks, and TKIs was reported to be moderate (1-10%), and recommended for HBV prophylaxis as well. However, the strength of such recommendation is weak.
It is advisable to screen all patients who are planned for TKI treatment. Suitable screening tests include HBV DNA, HBsAg, and ALT. It is important to identify patients with previous HBV infection, as evidenced by hepatitis B serological markers, i.e. positive hepatitis B core antibody (anti-HBc), with negative HBsAg, with or without detectable hepatitis B surface antibody (HBsAb). Nevertheless, running all tests may not be feasible due to costs and availability of testing equipment. Therefore, if unavailable, testing patients using only ALT and HBsAg, or even ALT alone would suffice, as the detection of HBVr can be deduced from ALT/HBsAg levels alone.
For HBsAg positive patients, prophylaxis against Hepatitis B reactivation should be started as HBVr may happen any time after commencement of TKIs. There is no clear answer in the literature as to which antiviral should be used. However, entecavir was most commonly used in the studies (other antivirals used include tenofovir and lamivudine). The duration of prophylaxis was also not mentioned in the literature.
CONCLUSION:
As the definition of hepatitis B reactivation includes a rise of ALT (2- to 3-fold), HBV DNA (newly detected to 10-fold increase) and seroconversion in HBsAg negative patients, all patients with CML should have baseline values of ALT, hepatitis B serology as well as HBV DNA before starting TKI treatment and should be followed up with repeat values of these 3 parameters. However, due to the cost and limited availabilities of these tests, we suggest following up at least ALT levels (preferably with HBsAg levels) before and after treatment with TKIs. In patients with risk of HBVr (i.e. positive HBsAg on screening, as well as those with resolved HBV infection), prophylaxis is important.
Based on currently available evidence, we recommend HBV prophylaxis for patients with positive HBsAg receiving TKIs to be given from the start of TKI treatment, and continued for at least 1 year after the commencement of TKIs, if feasible. In the event that this is not affordable, it is advisable to monitor markers of reactivation, as mentioned above (i.e. ALT, HBsAg, HBV DNA, whichever is available). For those with resolved HBV infection (i.e. positive anti-HBc and negative HBsAg), we recommend monitoring for signs of HBVr during the treatment duration.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal