The detection and quantification of BCR-ABL1 transcripts play a key role in therapy management of chronic myeloid leukemia (CML) patients treated with tyrosine kinase inhibitors (TKIs). Reverse transcription quantitative PCR (RT-qPCR) is considered the gold standard strategy for monitoring of minimal residual disease (MRD). Recently, digital PCR (dPCR) was introduced as a new quantification method based on determination of absolute target sequence quantity. In connection with the deep molecular response (MR) assessment and the option of TKIs treatment cessation, the dPCR has a potential of high sensitivity, robustness and accuracy of BCR-ABL1 transcripts detection. The aim of present work was to compare the results of two standardized RT-qPCR methods with RT-dPCR detection in clinical samples of CML patients with different BCR-ABL1 fusion transcripts level.
In total, 62 peripheral blood samples of CML patients with the level of BCR-ABL1 transcripts (international scale, IS) between 100% and undetectable were enrolled. The samples were analyzed by three methods: (1) CE IVD Xpert BCR-ABL Ultra Kit (GeneXpert, Cepheid), (2) conventional RT-qPCR assay according to ELN guidelines and certified by EUTOS for the level of MR4.5 BCR-ABL1 detection (ABI7300, Applied Biosystems), and (3) RT-dPCR using CE IVD QXDx BCR-ABL %ID Kit (Bio-Rad) on QX200 Droplet Digital PCR System (Bio-Rad). All steps were performed according to manufacturer´s instructions. A linear regression analysis and an overall reporting bias analysis using the Bland-Altman test were used for comparison of the methods.
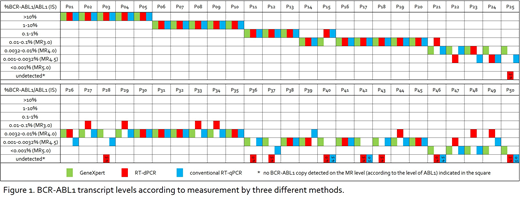
Based on the results of GeneXpert as the determined routine method for BCR-ABL1 transcripts quantification, the samples were divided into two groups: 50/62 samples were scored as BCR-ABL1 positive (the first group), while 12/62 samples were scored as BCR-ABL1 undetectable (the second group). The first group of GeneXpert positive samples was analyzed using both conventional RT-qPCR and RT-dPCR (both in duplicates). The median of the sum of ABL copies per sample was 180,511 copies (range 56,466 - 536,453) by RT-qPCR vs. 39,960 (range 14,360 - 71,690) by RT-dPCR. Both conventional RT-qPCR and RT-dPCR did not detect any BCR-ABL1 transcript in 4/50 samples. In addition, RT-dPCR did not detect any BCR-ABL1 transcript in 5 other samples (Figure 1). Linear regression analysis showed good correlation between both sets of assays: GeneXpert vs. RT-dPCR (R2=0.965, N=41) and conventional RT-qPCR vs. RT-dPCR (R2=0.977, N=41). The slopes of regression curve were not significantly different from the value of 1 for both sets of the compared assays (1.02; 95% CI, range 0.95 - 1.08 and 1.02; 95% CI, range 0.97 - 1.08, respectively). In addition, an overall bias (Bland-Altman analyses) was -0.05 (GeneXpert vs. RT-dPCR) and -0.12 (conventional RT-qPCR vs. RT-dPCR) suggesting good concordance in % IS reporting between RT-dPCR and two standardized quantitative BCR-ABL1 assays. The evaluation of MR level was identical by all three methods in 26/50 samples. The comparison of two methods revealed consistent MR level by GeneXpert vs. RT-dPCR in 29/50 samples and by conventional RT-qPCR vs. RT-dPCR in 28/50 samples. The RT-dPCR scored 12/50 samples (GeneXpert vs. RT-dPCR) and 13/50 samples (conventional RT-qPCR vs. RT-qPCR) to the different level of MR compared to standardized RT-qPCR methods. The second group of samples (12/62) undetectable for BCR-ABL1 transcripts according to GeneXpert was analyzed using conventional RT-qPCR and RT-dPCR in tetraplicates to reach higher sensitivity. The BCR-ABL1 negativity was confirmed in 11/12 samples. One sample was BCR-ABL1 positive by both the conventional RT-qPCR (0.0008% IS) and RT-dPCR (0.0013% IS).
We showed that the results of quantitative detection of BCR-ABL1 transcripts (% IS) obtained by the CE IVD RT-dPCR kit are in good correlation with the results of both standardized RT-qPCR methods and all three methods provide comparable sensitivity. RT-qPCR method yielded higher number of copies of control ABL gene per sample compared to RT-dPCR although the input amount of RNA into the RT reaction was the same. The MR level evaluation of RT-dPCR vs. both RT-qPCR methods was identical to the level MR3.0 (category >0.01% IS), while for samples in deep MR (category ≤ 0.01% IS, MR4.0 and below) revealed partial difference in categorization into the individual MR levels.
Supported by MH CZ - DRO (FNBr, 65269705).
Zackova:Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Angelini: Consultancy; Incyte: Consultancy. Mayer:AOP Orphan Pharmaceuticals AG: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal