Background: Some patients receiving a tyrosine kinase inhibitor (TKI) for chronic phase (CP) Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) experience drug intolerance and require switching to an alternative TKI. Bosutinib, a TKI approved for newly diagnosed CP Ph+ CML and Ph+ CML resistant/intolerant to prior therapy, has a distinct adverse event (AE) profile vs other TKIs used to treat Ph+ CML.
Methods: The ongoing phase 4 BYOND study (NCT02228382) is further evaluating efficacy and safety of bosutinib for CML resistant/intolerant to prior TKIs. Patients with Ph+ CP CML (n=156) previously treated with imatinib, dasatinib, and/or nilotinib received bosutinib (starting dose 500 mg once daily). Cross-intolerance (AEs leading to permanent discontinuation of both prior TKI and bosutinib), recurrent AEs (grades 1/2 or 3/4), and bosutinib dose modifications due to recurrent AEs were assessed across AEs and AE clusters. This analysis was based on ≥1 year after the last enrolled patient (median treatment duration 23.7 months [range 0.2-42.2]).
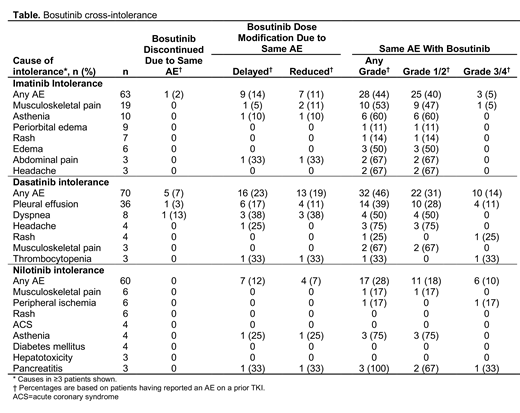
Results: Of 141, 95, and 79 patients who received prior imatinib, dasatinib, or nilotinib, respectively, 63 (45%), 70 (74%), and 60 (76%) were intolerant and discontinued treatment due to an AE as the primary reason. 1 (2%) imatinib-intolerant and 5 (7%) dasatinib-intolerant patients had cross-intolerance with bosutinib; no cross-intolerance with bosutinib was reported for the 60 nilotinib-intolerant patients (Table). 15 patients discontinued >1 TKI due to the same AE. Of these, only 1 with prior imatinib and dasatinib intolerance due to anemia (below), was cross-intolerant to bosutinib. No deaths occurred due to cross-intolerance to bosutinib.
Imatinib-intolerant: The most common cause of imatinib intolerance was musculoskeletal pain in 19 patients. Of these, 9 patients experienced grade 1/2 recurrence and 1 had a grade 3/4 AE. There was no cross-intolerance with bosutinib due to musculoskeletal pain. 6 patients were intolerant due to edema, which recurred as grade 1/2 in 3 bosutinib-treated patients. In 2 patients with intolerance due to diarrhea, both had grade 1/2 diarrhea with bosutinib. 2 patients were imatinib-intolerant due to neutropenia or thrombocytopenia (n=1 each) and experienced grade 3/4 recurrence with bosutinib. 1 patient was cross-intolerant to bosutinib due to anemia.
Dasatinib-intolerant: The most common reason for prior dasatinib intolerance was pleural effusion. Of 36 patients, 10 (28%) had recurrence of grade 1/2 AE and 4 (11%) had grade 3/4 AE with bosutinib that caused dose delay and reduction in 6 (17%) and 4 (11%) patients, respectively. 1 patient had cross-intolerance with bosutinib due to pleural effusion. Of 8 patients dasatinib-intolerant due to dyspnea, 1 had cross-intolerance with bosutinib (this patient also developed pleural effusion with dasatinib). Of 2 patients with dasatinib intolerance due to pulmonary hypertension, 1 had grade 1/2 and 1 had grade 3/4 recurrence with bosutinib, leading to cross-intolerance in 1 patient. 2 other dasatinib-intolerant patients experienced cross-intolerance due to anemia and nausea (n=1 each). Grade 3/4 recurrence of thrombocytopenia was experienced in 1 of 3 patients with prior dasatinib-intolerance. 1 patient with dasatinib intolerance due to diarrhea had grade 3/4 diarrhea with bosutinib that was managed with dose delay/reduction.
Nilotinib-intolerant: 6 patients discontinued nilotinib due to peripheral ischemia, of whom 1 experienced grade 3/4 recurrence with bosutinib. None of the 4 patients with nilotinib intolerance due to acute coronary syndrome had recurrence with bosutinib. Of 3 patients with prior intolerance due to pancreatitis, 1 had grade 3/4 recurrence with bosutinib. 2 patients had recurrent diarrhea with bosutinib, both grade 1/2. There were no bosutinib dose reductions, delays, or recurrence of AEs in patients with prior nilotinib intolerance due to rash, hematologic AEs, hepatotoxicity, or metabolic disorders.
Conclusions: Incidence of cross-intolerance, dose delay, or dose reduction with bosutinib in patients intolerant to prior TKIs was low. Despite recurrence of certain same grade 1/2 or grade 3/4 AEs that caused prior TKI intolerance, these were manageable and patients were able to remain on bosutinib. These findings support the use of bosutinib in patients with CP Ph+ CML intolerant to prior TKI treatment.
Gjertsen:Haukeland University Hospital / University of Bergen: Employment; ERA PerMed: Research Funding; BerGenBio: Consultancy; Astellas: Consultancy; BerGenBio AS: Membership on an entity's Board of Directors or advisory committees; KinN Therapeutics AS: Equity Ownership; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy; ACTII AS: Equity Ownership; Seattle Genetics: Consultancy; Research Council of Norway: Research Funding; EU Horizon 2020: Research Funding; The Norwegian Cancer Society: Research Funding; Helse Vest Health Trust: Research Funding. Hochhaus:Pfizer: Research Funding; BMS: Research Funding; Incyte: Research Funding; MSD: Research Funding; Novartis: Research Funding. Rosti:BMS: Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Watts:Celgene: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees. Ortí:Bristol-Myers Squibb: Consultancy, Other: Travel Expenses, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Other: Travel Expenses, Speakers Bureau. le Coutre:Bristol-Myers Squibb: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Incyte: Honoraria, Speakers Bureau. Leip:Pfizer: Employment, Equity Ownership. Viqueira:Pfizer Inc: Employment, Equity Ownership. Cortes:Biopath Holdings: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding. Giles:Novartis: Consultancy; Epigene Therapeutics Inc: Consultancy, Other: leadership, stock/other ownership ; Actuate Therapeutics Inc: Employment. Gambacorti-Passerini:Pfizer: Honoraria, Research Funding; Bristol-Meyers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal