Background: Neurolymphomatosis (NL) is a rare (1.9%) extranodal manifestation of non-Hodgkin lymphoma characterized by infiltration of malignant lymphoma cells into the peripheral nervous system. The initial presentation can be an extranodal lesion during the course of disease progression (primary NL) or recurrence (secondary NL). Diagnosis is often difficult as clinical symptoms of NL are nonspecific and varied. Although nerve biopsy of the affected nerve remains the gold standard for diagnosis, it is often unfeasible due to possible irreversible neurological deficits and patchy distribution of the NL lesions. The prognosis and optimal therapeutic strategy for NL are poorly understood because of its rarity. To clarify the clinical features and characteristics of NL, we retrospectively analyzed 22 cases of NL with emphasis on diagnosis and treatment. This is the largest series of NL cases as a single-institution report.
Method: We reviewed the medical records of patients admitted to the Kameda Medical Center from April 2006 to April 2018. Diagnosis of NL required clinical symptoms and neurological findings related to the cranial, peripheral or spinal nerves with either histological confirmation of malignant lymphoma cells within the peripheral nerve, nerve root/plexus, or cranial nerve or contrast-enhanced magnetic resonance imaging (MRI) demonstration of nerve enhancement and/or enlargement of the peripheral nerve or nerve root that was also demonstrated by uptake of FDG on 18F-FDG-PET/CT (PET/CT) and/or whole-body diffusion-weighted MRI (WBDWI). We surveyed therapeutic contents and analyzed prognosis.
Results: Over the past 12 years, 1181 patients with non-Hodgkin lymphoma were admitted to our hospital; 22 patients (1.8%) were diagnosed with NL (12 men and 10 women; median age: 73.0 years, range: 46-85 years). Lymphoma subtype included DLBCL in 20 patients, mantle cell lymphoma in 1 patient, and peripheral T-cell lymphoma in 1 patient. Six patients (27.2%) were diagnosed with primary NL and 16 patients (72.8%) were diagnosed with secondary NL. All the patients with secondary NL showed no prominent lymphadenopathy. Among the 20 DLBCL patients, 6 (30%) had intravascular large B-cell lymphoma and 10 (50%) had CD5-positive lesions. In 9 patients, NL lesions were evaluated by both PET/CT and contrast enhanced MRI with WBDWI. All the patients were positive by contrast-enhanced MRI, and 7 (77.7%) patients were positive by WBDWI, which was the same rate observed with PET/CT. Cerebrospinal fluid cytology was positive in 13 cases (59%). The serum sIL2R levels were significantly lower in patients with secondary NL than in patients with primary NL (614 U/mL vs 2024 U/mL, p = 0.02), but the serum LDH levels were not significantly different between patients with primary and secondary NL (283 U/L vs 244 U/L, p = 0.7).
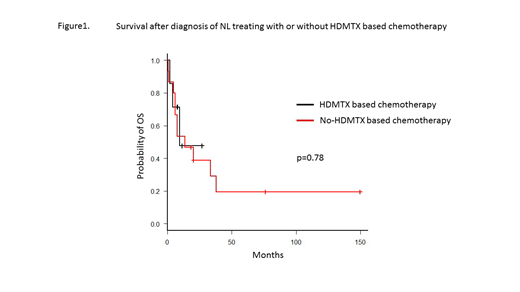
Fifteen patients received high-dose methotrexate (HD-MTX)-based chemotherapy, with a cumulative dose of 1800 mg/m2 to 16000 mg/m2. Despite initial responses, rapid disease progression occurred in all patients. The median survivals after diagnosis of NL in patients with and without HD-MTX-based chemotherapy were 13.4 months and 9.4 months, respectively (p = 0.78). Neurological symptoms responded promptly in 5 patients receiving involved nerve irradiation, but not in patients receiving HD-MTX-based chemotherapy. Three patients received autologous stem cell transplantation (auto-SCT); 1 patient survived for more than 12 years free of disease, one survived for 22 months, and one patient relapsed and died 7 months after auto-SCT.
Fourteen patients (63.6%) died due to progressive NL. The overall survivals from the diagnosis of the hematologic malignancy and the development of NL were 19 months (range: 11.0-47.0 months) and 13.4 months (range: 5.9-37.7 months), respectively.
Conclusion: NL is an extremely rare manifestation of malignant lymphoma. Pathological diagnosis with nerve biopsy is often avoided because of concern over permanent neurological deficit; however, imaging techniques, especially WBDWI, showed promising sensitivity for the detection of NL lesions. Prognosis remains extremely poor once NL has developed despite the administration of high-dose MTX and rituximab-containing chemotherapy with or without auto-SCT.
Matsue:Celgene: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Ono Pharmaceutical: Honoraria; Novartis Pharma K.K: Honoraria; Janssen Pharmaceutical K.K.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal