Background: Asymptomatic proximal deep-vein thrombosis (ASxDVT) is an efficacy endpoint that has been used for several decades in clinical trials evaluating thromboprophylaxis with anticoagulants. Previous studies have suggested the finding of ASxDVT on routine ultrasonography is associated with increased subsequent all-cause mortality (ACM). We evaluated the relationship between ASxDVT and subsequent ACM using the data from the MAGELLAN study (NCT00571649), a randomized clinical trial that evaluated rivaroxaban compared with enoxaparin followed by placebo for the prevention of venous thromboembolism (VTE) in acutely ill medical patients in which routine compression ultrasonography was performed at Day 10 and at Day 35. Patients were followed up through 90 days.
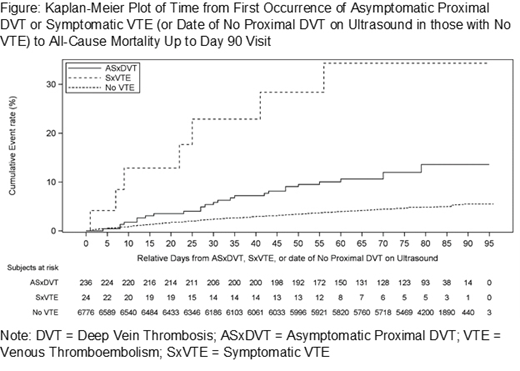
Methods: A post hoc analysis was performed using patients who received at least one dose of study drug and had an adequate ultrasound result at Day 10 or Day 35 (modified intent-to-treat population, mITT). Patients were categorized into one of three mutually exclusive groups: (1) those without VTE; (2) those with symptomatic VTE (SxVTE); and (3) those with an ASxDVT. If patients had an ASxDVT followed by an SxVTE event, they were categorized into the ASxDVT group. Baseline covariates (age, sex, race, BMI, diabetes, creatinine clearance, heart failure, acute ischemic stroke, acute infectious disease, inflammatory disease, acute respiratory insufficiency, history of VTE, history of cancer, history of anemia and assigned treatment group) were tested for association with ACM (p<0.05). A Cox proportional hazards model, which included the VTE variable and significant baseline covariates (history of cancer, heart failure, acute ischemic stroke, inflammatory disease, acute respiratory insufficiency, BMI, history of anemia) was used to compare the risk of ACM through the Day 90 visit in subjects who had ASxDVT, SxVTE or neither event. A Kaplan-Meier plot was used to display the time from the first VTE event (or time of the first ultrasound in those without an event) to ACM in the three groups.
Results: In total, 7036 patients were included in the combined mITT Day 10 /mITT Day 35 analysis set. Of these, 6776 (96.3%) were included in the no VTE group, 236 (3.3%) in the ASxDVT group and 24 (0.34%) in the SxVTE group. Compared with no VTE (incidence of ACM=4.8%), both ASxDVT (11.4%, HR 2.31, 95%CI 1.52-3.51, p<0.0001) and SxVTE (29.2%, HR 9.42 95%CI 4.18-21.20, p<0.0001) were associated with a significant increase in ACM. The Kaplan-Meier plot shows early and continued separation in both groups compared with the group without a VTE event.
Limitations: The analysis was post hoc and the groups were defined post-randomization. Adjustment was done for baseline variables that were significantly associated with ACM but may not fully compensate for potential post-randomization differences.
Conclusion: These results suggest that the finding of an asymptomatic proximal DVT on routine compression ultrasonography is associated with a significantly higher ACM in acutely ill medical patients. A similar relationship was found for SxVTE and ACM, which supports the validity of ASxDVT as an indicator of clinically important disease and an appropriate endpoint for thromboprophylaxis trials in the medically ill.
Raskob:Janssen R&D, LLC: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Novartis: Consultancy; Boehringer Ingelheim: Consultancy; Eli Lilly: Consultancy; Anthos: Consultancy; Tetherex: Consultancy; Portola: Consultancy; BMS: Consultancy, Honoraria; Bayer Healthcare: Consultancy, Honoraria. Spyropoulos:Daiichi Sankyo: Consultancy; Boehringer Ingelheim: Consultancy, Research Funding; Portola: Consultancy; Bayer Healthcare: Consultancy; ATLAS (Colorado Prevention Center): Consultancy; Janssen R&D, LLC: Consultancy. Cohen:Lifeblood: Other: advisor to Lifeblood: the thrombosis charity and is the founder of the European educational charity the Coalition to Prevent Venous Thromboembolism; AbbVie: Consultancy; Aspen: Consultancy, Speakers Bureau; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy; Guidepoint Global: Consultancy; Boston Scientific: Consultancy; Portola: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ONO: Consultancy, Membership on an entity's Board of Directors or advisory committees; Medscape: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Boehringer-Ingelheim: Consultancy, Speakers Bureau; McKinsey: Consultancy; Johnson and Johnson: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL Behring: Consultancy; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GlaxoSmithKline: Consultancy, Speakers Bureau; GLG: Consultancy; Leo Pharma: Consultancy; Navigant: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Temasek Capital: Consultancy; TRN: Consultancy; UK Government Health Select Committee: Other: advised the UK Government Health Select Committee, the all-party working group on thrombosis, the Department of Health, and the NHS, on the prevention of VTE; ACI Clinical: Consultancy. Weitz:Novartis: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Daiichi-Sankyo: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Boehringer Ingelheim: Consultancy, Honoraria; Bayer Healthcare: Consultancy, Honoraria; Janssen R&D, LLC: Consultancy; Pfizer: Consultancy, Honoraria; Portola: Consultancy, Honoraria. Ageno:Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: travel support; Aspen: Membership on an entity's Board of Directors or advisory committees, Other: travel support; Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees, Other: conference and travel support; Portola: Membership on an entity's Board of Directors or advisory committees, Other: travel support; Bayer: Membership on an entity's Board of Directors or advisory committees, Other: research support,travel support ; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees, Other: travel support; BMS Pfizer: Other: travel support. De Sanctis:Bayer US LLC: Employment, Equity Ownership. Lu:Janssen R&D, LLC: Employment, Equity Ownership. Xu:Janssen R&D, LLC: Employment, Equity Ownership. Albanese:Janssen Research and Development LLC: Employment, Equity Ownership. Sugarmann:Janssen Research and Development LLC: Employment, Equity Ownership. Lipardi:Janssen Research and Develompent: Employment, Equity Ownership. Spiro:Bayer U.S. LLC: Employment, Equity Ownership. Barnathan:Janssen Research and Development LLC: Employment, Equity Ownership.
Rivaroxaban is a factor Xa inhibitor and is currently under review by the FDA for thromboprophylaxis in patients with acute medical illness
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal