Background: The combination of methotrexate, temozolomide, and rituximab (MT-R) as per CALGB 50202 is a commonly used induction regimen for patients with primary central nervous system lymphoma (PCNSL). Achieving a high level of serum exposure to methotrexate (MTX) is important for therapeutic penetration of the central nervous system (CNS). However, in practice, the dose of MTX is often reduced from 8g/m2 due to age, renal dysfunction and co-morbidities. Thus, we performed a retrospective analysis of patients with PCNSL treated with MT-R at the University of Iowa to assess usual clinical practice in this disease, as well as outcomes associated with dose intensity of MTX and area-under-the-curve (AUC) exposure to MTX.
Methods: All patients with PCNSL treated at University of Iowa from July 2013 to March 2019 were identified from a prospectively collected institutional cohort and via retrospective review of pathology records. Newly diagnosed, immunocompetent PCNSL patients with diffuse large B cell lymphoma (DLBCL) histology receiving at least one dose of methotrexate were included. Baseline patient data were obtained via retrospective review of the medical record. Population pharmacokinetic modelling was performed using a nonlinear-mixed effect modeling approach to estimate the pharmacokinetic parameters of MTX in each patient and each cycle of therapy. Further model simulation was performed to estimate areas under the curve (AUC) of MTX for each dose of therapy. For each patient, the average dose of MTX (avgMTX) and estimated AUC (avgAUC) received across all treatments was calculated. Toxicities were retrospectively assessed and graded according to CTCAE v5.0. Imaging data was reviewed and response to treatment reported using the criteria described by Abrey et al. (JCO 2005 23:22 5034-43). Survival probabilities were estimated and plotted using the Kaplan-Meier method. Survival differences were evaluated with the log-rank test. Statistical analysis was performed using SAS v9.4.
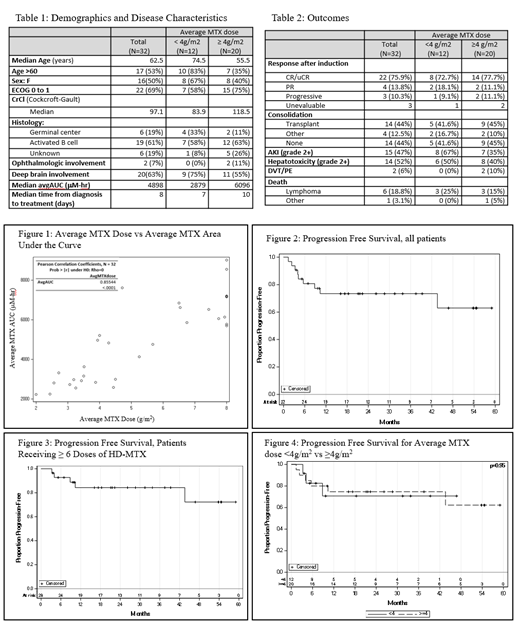
Results: Thirty-two patients were identified for inclusion. Baseline demographics and disease characteristics are shown in table 1. Age was ≥ 60 in 53%. Median baseline creatinine clearance was 97.1 ml/min. Eastern Cooperative Oncology Group (ECOG) performance status was ≤ 1 in 69% of patients. Median time from biopsy to treatment initiation was 8 days (range 2-39 days). Overall, 16% of patients received the maximum 8 g/m2. The mean dose of average MTX was < 4 g/m2 in 12 patients (37.5%) and was ≥ 4 g/m2 in 20 patients (63.5%) (Table 1). The correlation between avgMTX and avgAUC is shown in figure 1 (Pearson co-efficient 0.85, p-value <0.01).
Median follow up for the cohort was 22 months. Three total patients were unevaluable for response to induction: two patients with poor baseline functional status died shortly after beginning MT-R therapy and one patient completed therapy but had not yet undergone response assessment MRI imaging. At the end of induction therapy, the rate of complete remission (CR + uCR) was 75.9%, partial response (PR) was 13.8%, and progressive disease was 10.3% (table 2). The progression free survival at 24 months (PFS24) was 73% (figure 2). PFS24 for patients receiving ≥6 doses of methotrexate was 84% (figure 3). Among patients receiving an average dose of MTX < 4g/m2, the rate of CR/uCR/PR was 90.8%, and PFS24 was 71%. Among patients receiving an average dose of MTX ≥ 4g/m2, the rate of CR/uCR/PR was 88.8%, and PFS24 was 75% (figure 4).
The incidence of acute kidney injury (grade 2+) was 35% and 67% in the ≥ 4g/m2 and < 4g/m2 avgMTX dose groups, respectively. In total, 7 of 32 (21.9%) of patients died over a median follow-up time of 22 months. The cause of death was PCNSL in 6 patients, and bowel perforation in 1 patient (table 2).
Conclusions: Although few patients received the maximum dose of methotrexate (8g/m2), the progression free survival at 24 months was comparable to prior clinical trial experience (CALGB 50202). Despite a range of ages and creatinine clearances, the average dose of methotrexate and average AUC correlated well. Though subset analyses are limited by sample size, patients receiving MT-R with average MTX doses < 4g/m2 and ≥ 4g/m2 each had excellent outcomes in terms of response rate and progression free survival.
Farooq:Kite Pharma: Research Funding; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal