Introduction: Burkitt lymphoma (BL) has become an AIDS-defining disease by Centers for Disease Control (CDC) definition since 1993, emphasizing the strong relationship between this subtype of non-Hodgkin Lymphoma (NHL) and the Human Immunodeficiency Virus (HIV) infection. Although it is widely recognized that the HIV-associated BL represents a distinct entity and a more difficult-to-treat disease, reported results from developed countries seem to corroborate that these regimens do not need to be tailored to the HIV-positive population. There is no available data on outcomes of this population in developing countries. In this study, we report a real-world cohort of adult HIV-associated BL patients in Brazil.
Methods: This is a retrospective multicenter cohort encompassing 4 academic hospitals in Brazil. Ethics approval for the study was obtained in all centers. Patients were treated according to their local protocol, which included Hyper-CVAD, CODOX-M IVAC or CHOP-like regimens, on a clinical judgement basis. Further reductions in the dose could be done at discretion of physician in case of excessive toxicity. For all patients who were not using cART, HIV-directed therapy was promptly started and administered along with chemotherapy. A cytoreductive regimen could be administered prior to the actual regimen to minimize the risk of tumor lysis. Survival analysis was performed using the Kaplan-Meier method and log-rank test for comparison. Cumulative incidence of relapse (CIR) was calculated using death as a competitor.
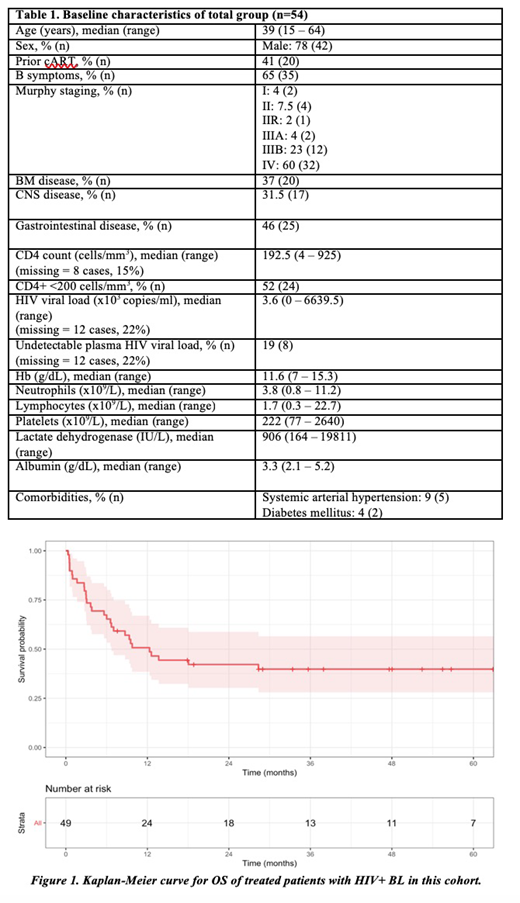
Results: A total of 54 patients with HIV-associated BL were included in this analysis. Median age was 39 years (range, 15 - 64) and the vast majority were male (78%). 41% of patients were using cART prior to the BL diagnosis. Advanced disease was found in 86% of patients. CNS disease was observed in 31%. Regarding the immune status at the presentation, 52% had CD4+ T-cells count lower than 200 cells/mm3 and 19% had undetectable levels of HIV viral load. Other features are summarized in Table 1. Five patients died before starting any regimen due to sepsis. Among the 49 patients, most were treated with a modified Hyper-CVAD (26/49, 53%), CODOX-M IVAC (9/49, 18%) and a CHOP-like regimen (8/49). Only 8/49 (16%) patients received rituximab in upfront therapy. Radiotherapy was used in 4/49 patients (2 cranial irradiation, 1 in initial bulky site and 1 in testicles). A treatment-related mortality of 38.7% (95% confidence interval [CI] 25.5 - 53.7) was found and the complete response (CR) rate was 44.9% (95% CI 30.9 - 59.6). Primary refractory disease was found in 14%. Regarding the patients who achieved a CR at the end of treatment, 3-year CIR was 6.2% (95% CI 1.6 - 15.7%). There was no recommended regimen for salvage, and most patients received an alternate classic regimen for BL if feasible. Only one relapsed patient proceeded to autologous stem-cell transplantation, but eventually died after a second relapse. Median follow-up time was 4.4 years. A 4-year OS of 36.1% (95% CI 25.2 - 51.8) for total cohort and 39.8% (95% CI 28.1 - 56.5) for those patients who received any regimen was calculated (Figure 1). A univariate analysis for CR and OS did not show any statistically significant difference regarding sex, age, staging, CD4+, viral load, prior cART, extranodal infiltration, blood counts, lactate dehydrogenase (LDH), employed regimen or use of rituximab. All relapsed and primary refractory patients eventually died in our cohort. Aside from those patients who died from disease progression per se, remaining patients died from infections (24/34), despite intensive antimicrobial prophylaxis and associated cART.
Conclusion: Outcomes of HIV-positive BL population are scarcely reported worldwide. While HIV patients seem to have a higher relapse rate in other studies, our early mortality and overall survival were far worse than those reported in developed countries and with more selected cohorts. In comparison with literature, our patients experienced higher toxicity with classic regimens and higher refractoriness rate. Therefore, these findings warrant further cooperative multicenter studies in developing countries, aiming at improving supportive care and optimizing regimens locally feasible.
Delamain:Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal